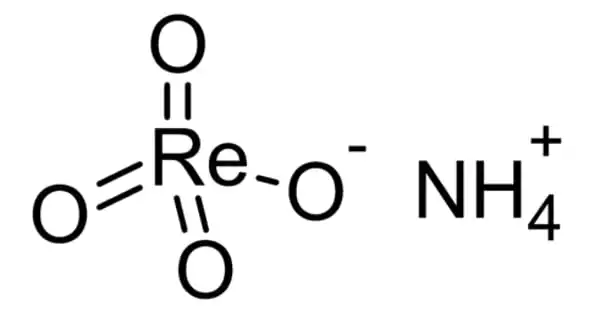The ammonium salt of perrhenic acid, NH4ReO4, is ammonium perrhenate (APR). It is the most commonly traded form of rhenium. It is a white salt that is soluble in ethanol and water and only slightly soluble in NH4Cl. It was first described shortly after rhenium was discovered. It is an intermediate product in the rhenium extraction process that allows for the production of rhenium powder.
Properties
- Molecular Weight: 268.24
- Appearance: White Powder
- Melting Point: N/A
- Boiling Point: N/A
- Density: 3.97 g/cm3
- Solubility in H2O: 6.2 g/100 mL (20 °C)
- Crystal Phase / Structure: scheelite
- Exact Mass: 268.969786
Structure
APR’s crystal structure is similar to that of scheelite, except that the atomic cation is replaced by ammonium. This motif is followed by pertechnetate (NH4TcO4), periodate (NH4IO4), tetrachlorothallate (NH4TlCl4), and tetrachloroindate (NH4InCl4). On cooling, it goes through a molecular orientational ordering transition without changing its space group, but with a highly anisotropic change in the form of the unit cell, resulting in the uncommon attribute of having a positive temperature and pressure Re NQR coefficient. Hydrates are not provided by APR.

Preparation
Ammonium perrhenate can be made from almost any common rhenium source. Nitric acid can be used to oxidize the metal, oxides, and sulfides, and the resultant solution can be treated with aqueous ammonia. An aqueous solution of Re2O7 can also be treated with ammonia before crystallization.
Reactions
Ammonium perrhenate is weak oxidizer. It slowly reacts with hydrochloric acid:
NH4ReO4 + 6 HCl → NH4[ReCl4O] + Cl2 ↑ + 3H2O.
It is reduced to metallic Re upon heating under hydrogen:
2 NH4ReO4 + 7 H2 → 2 Re + 8 H2O + 2 NH3
Ammonium perrhenate decomposes to volatile Re2O7 starting at 250 °C. When heated in a sealed tube at 500 °C, It decomposes to rhenium dioxide:
2NH4ReO4 → 2ReO2 + N2 + 4 H2O
The ammonium ion can be displaced with some concentrated nitrates e.g. potassium nitrate,, silver nitrate, etc.:
NH4ReO4 KNO3 → KReO4 ↓ + NH4NO3
It can be reduced to nonahydridorhenate with sodium in ethanol:
NH4ReO4 + 18Na + 13C2H5OH → Na2[ReH9] + 13NaC2H5O + 3NaOH + NH3•H2O.