Sodium thioantimoniate, also known as sodium antimony thiolate or sodium thioantimonite, is a chemical compound with the formula Na3SbS4. It is a yellowish-green solid that is soluble in water. The nonahydrate of this chemical, Na3SbS4·9H2O, is known as Schlippe’s salt, named after Johann Karl Friedrich von Schlippe (1799–1867), These compounds are examples of sulfosalts. They were once of interest as species generated in qualitative inorganic analysis.
Sodium thioantimoniate is used in analytical chemistry as a reagent for the detection of gold, platinum, and palladium. It is also used in the production of pigments, as well as in the manufacture of antimony compounds. Additionally, it has been studied for its potential use as a semiconductor material in solar cells. However, it should be handled with care as it is toxic and can be a skin and eye irritant.
Properties
Sodium thioantimoniate is a yellow to orange crystalline powder or solid. It is insoluble in water, but soluble in acids and alkalis. It is stable under normal conditions, but can decompose when exposed to moisture and air.
- Chemical formula: Na3SbS4 (anhydrous); Na3SbS4·9H2O (nonahydrate)
- Molar mass: 272.13 g·mol−1 (anhydrous); 434.27 g·mol−1 (nonahydrate)
- Appearance: Yellow crystals
- Density: 1.806 g/cm3, solid
- Melting point: 87 °C (189 °F; 360 K)
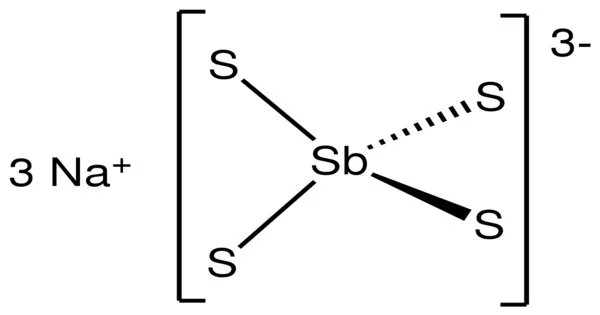
Structure
The nonahydrate consists of the tetrahedral tetrathioantimonate(V) anions SbS3-4 and sodium cations Na+, which are hydrated. The Sb-S distance is 2.33 Å. Related salts are known for different cations including ammonium and potassium. The anhydrous salt is a polymer with tetrahedral Na and Sb sites.
Preparation
Sodium tetrathioantimonate nonahydrate is prepared by the reaction of “antimony trisulfide”, elemental sulfur, and aqueous sulfide source.
3 Na2S + 2 S + Sb2S3 + 18 H2O → 2 Na3SbS4·9H2O
The Na2S can be generated in situ by the reaction of sodium hydroxide and S (co-generating sodium sulfate):
Sb2S3 + 8 NaOH + 6 S → 2 Na3SbS4 + Na2SO4 + 4 H2O
Charcoal can also be used to reduce sulfur.
The required antimony trisulfide is prepared by treatment of Sb(III) compounds with sulfide sources:
2 SbCl3 + 3 H2S → Sb2S3 + 6 HCl
Reactions
The hydrate dissolves in water to give the tetrahedral SbS43- ion. The salt gives “quinsulfide antimony,” upon acidification:
2 Na3SbS4 + 6 HCl → Sb2S5 + 6 NaCl + 3 H2S
Application
Sodium thioantimoniate is primarily used as a reagent in analytical chemistry and as a precursor for the synthesis of other antimony compounds. It has semiconducting properties, which makes it useful in applications such as solid-state electrolytes and photovoltaic devices. It is considered to be toxic and can cause irritation to the skin, eyes, and respiratory system if inhaled or ingested.