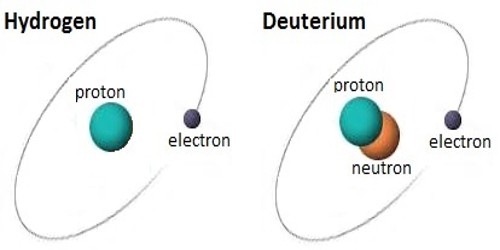
Deuterium is an isotope of hydrogen, the first element. It has an atomic weight of 2.014. It is a stable atomic species found in natural hydrogen compounds to the extent of about 0.0156 percent. It is composed of one proton, one electron, and one neutron. It was discovered by Harold C. Urey, professor of chemistry at Columbia University in the winter of 1931.
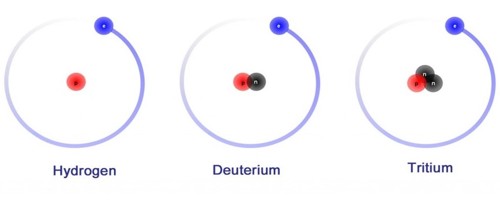
Deuterium has one proton and one neutron. Hydrogen does not have a neutron, only a proton. Another isotope of hydrogen, tritium, has two neutrons. It is an isotope of hydrogen. The chemical symbol for Deuterium is 2H but D is also used often. Deuterium gas is one form of naturally occurring pure hydrogen. Its chemical formula is written as either 2H2 or as D2. Pure deuterium gas is rare. It is a colorless, odorless gas. It is easily ignited.
Two deuterium atoms combined with an oxygen atom is sometimes called “heavy water.” This is because it is like water (H2O), but heavier because deuterium has one more neutron in its nucleus. Heavy water is sometimes used in nuclear reactors. It is also used as a solvent for NMR spectroscopy. This is because it will dissolve the sample, like normal water, but it will not be detected by the magnet in a 1H NMR.
Deuterium is used as a tracer, in nuclear fusion reactors and to slow down neutrons in heavy water moderated fission reactors. It could be produced in a nuclear reactor, but the method is not cost-effective. It is not toxic but is a simple asphyxiate by the displacement of oxygen in the air.