Hydroxide
Definition
Hydroxide is a chemical compound containing one or more hydroxyl radicals (OH). The positively charged portion of the compound usually is the ion of a metal (e.g., sodium, magnesium, or aluminum), although it may be an organic group (e.g., guanidinium or tetramethylammonium). It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical.
An ion is an atom or molecule that does not have the same number of electrons as protons. Ions can be positively charged or negatively charged. Ions are positive if the number of protons is greater than the number of electrons, and it is negative if the number of protons is less than the number of electrons. Some metal hydroxides, such as those of zinc and lead, are amphoteric. Many inorganic substances which bear the word “hydroxide” in their names are not ionic compounds of the hydroxide ion, but covalent compounds which contain hydroxyl groups. A hydroxide attached to a strongly electropositive center may itself ionize, liberating a hydrogen cation (H+), making the parent compound an acid.
Hydroxide Ion
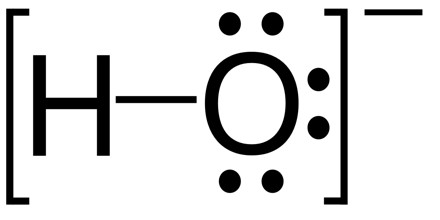
The hydroxide ion is a negatively charged molecule made up of one oxygen bonded to one hydrogen. When dissolved in water, the hydroxide ion is an incredibly strong base. In fact, according to the Arrhenius definition of a base, the presence of a hydroxide ion is what makes a chemical a base. A base is a chemical that has a high pH, tastes soapy, feels slippery and reacts well with acid.
In aqueous solution the hydroxide ion is a base in the Brønsted–Lowry sense as it can accept a proton from a Brønsted–Lowry acid to form a water molecule. It can also act as a Lewis base by donating a pair of electrons to a Lewis acid. In aqueous solution both hydrogen and hydroxide ions are strongly solvated, with hydrogen bonds between oxygen and hydrogen atoms.

Other hydroxide containing ionic compounds are fairly insoluble in water, like bright blue copper (II) hydroxide or the brown iron (II) hydroxide. While they may be toxic, these compounds don’t pose as much immediate danger as sodium hydroxide or potassium hydroxide.
In some cases the products of partial hydrolysis of metal ion, described above, can be found in crystalline compounds. The compound originally formulated as ZrOCl2·8H2O was found to be the chloride salt of a tetrameric cation in which there is a square of Zr4+ ions with two hydroxide groups bridging between Zr atoms on each side of the square and with four water molecules attached to each Zr atom.
Structure: The hydroxide ion appears to rotate freely in crystals of the heavier alkali metal hydroxides at higher temperatures so as to present itself as a spherical ion, with an effective ionic radius of about 153 pm. It displays cylindrical symmetry in hydroxides of divalent metals Ca, Cd, Mn, Fe, and Co. For example, magnesium hydroxide Mg(OH)2 (brucite) crystallizes with the cadmium iodide layer structure, with a kind of close-packing of magnesium and hydroxide ions.
Applications of Hydroxide
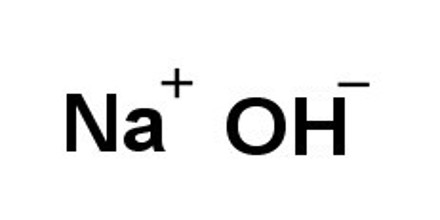
The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium, and cesium, are the strongest bases and the most stable and most soluble of the hydroxides. Sodium hydroxide, NaOH, also known as caustic soda or lye, is of great industrial importance. Calcium, barium, and strontium—all alkaline earth metals—form soluble hydroxides that are strong bases but are less stable than the alkali hydroxides. Of these, calcium hydroxide, Ca(OH)2, commonly known as slaked lime, is the most common. Hydroxides and other substances, such as oxides and sulfides, with these dual properties are called amphoteric.