The solubility of a substance depends on the physical and chemical properties of that substance. It is the maximum amount of a substance that will dissolve in a given amount of solvent at a specific temperature. In addition to this, there are a few conditions that can manipulate it. Temperature affects the solubility of both solids and gases, but the pressure only affects the solubility of gases. Temperature, pressure, and the type of bond and forces between the particles are few among them.
Factors Affecting Solubility –
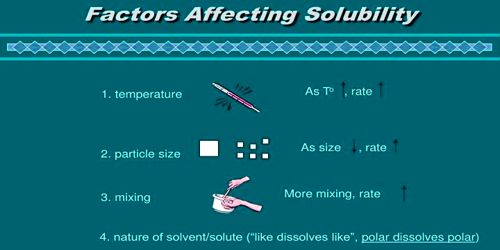
- Temperature:
The temperature has a direct effect on solubility. By changing the temperature we can increase the soluble property of a solute. Generally, water dissolves solutes at 20° C or 100° C. For the majority of ionic solids, increasing the temperature increases how quickly the solution can be made. Sparingly soluble solid or liquid substances can be dissolved completely by increasing the temperature. Temperature can also increase the amount of solute that can be dissolved in a solvent. But in the case of gaseous substance, temperature inversely influences solubility i.e. as the temperature increases gases expand and escapes from their solvent. For all gases, as the temperature increases, solubility decreases.
- Forces and Bonds:
Like dissolves in like. The type of intermolecular forces and bonds vary among each molecule. If we were to increase the surface area of a solid, then it would have been broken into smaller pieces. We would do this to increase how quickly the solute would dissolve in solution. The chances of solubility between two unlike substances are more challengeable than the like substances. If you were to dissolve sugar in water, a sugar cube will dissolve slower than an equal amount of tiny pieces of sugar crystals. For example, water is a polar solvent where a polar solute like ethanol is easily soluble.
- Pressure:
Pressure, affects the solubility of a gas in a liquid but never of a solid dissolving in a liquid. Gaseous substances are much influenced than solids and liquids by pressure. When pressure is applied to a gas that is above the surface of a solvent, the gas will move into the solvent and occupy some of the spaces between the particles of the solvent. When the partial pressure of gas increases, the chance of its solubility is also increased. When the gas pressure is decreased, the solubility of that gas is also decreased. A soda bottle is an example of where CO2 is bottled under high pressure. When you open a can of carbonated beverage, the pressure in the soda is lowered, so the gas immediately starts leaving the solution.
Information Source: