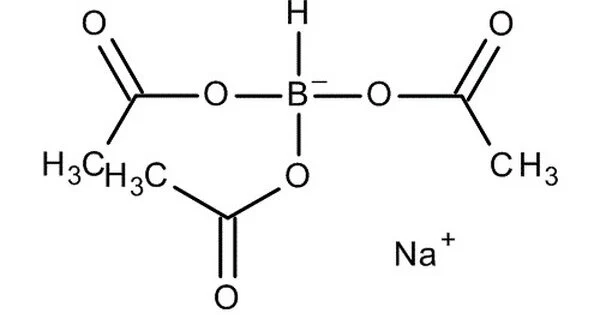
Sodium triacetoxyborohydride (STAB), also known as sodium triacetoxyhydroborate, is a chemical compound with the formula Na(CH3COO)3BH. It is used as a reducing agent in organic synthesis, as are other borohydrides. The protonolysis of sodium borohydride with acetic acid yields this colorless salt:
NaBH + 3 HO2CCH3 → NaBH(O2CCH3)3 + 3 H2
Sodium triacetoxyborohydride is a commonly used reducing agent in organic chemistry. It is a white crystalline powder and is soluble in polar solvents such as water and methanol.
Properties
It is a white to off-white crystalline powder that is soluble in water, methanol, and ethanol. The molecular weight of sodium triacetoxyborohydride is 252.05 g/mol. It is soluble in many organic solvents, such as dichloromethane, tetrahydrofuran (THF), and dimethyl sulfoxide (DMSO), but insoluble in water. It is a powerful reducing agent that can reduce a wide range of functional groups, including aldehydes, ketones, imines, and enamines, among others.
- Chemical formula: C6H10BNaO6
- Molar mass: 211.94 g·mol−1
- Appearance: White powder
- Density: 1.20 g/cm3
- Melting point: 116 to 120 °C (241 to 248 °F; 389 to 393 K) decomposes
- Solubility in water: decomposition
Comparison with related reagents
Sodium triacetoxyborohydride is a less harsh reducing agent than sodium borohydride or sodium cyanoborohydride. It is effective at reducing aldehydes but not most ketones. It is particularly well suited for reductive aminations of aldehydes and ketones. However, unlike sodium cyanoborohydride, triacetoxyborohydride does not readily hydrolyze and is not compatible with methanol. It reacts slowly with ethanol and isopropanol and can therefore be used with them.
Preparation
It is typically prepared by reacting sodium borohydride with acetic acid in the presence of boron trifluoride etherate as a catalyst. The resulting product is a complex of sodium, boron, and acetate ions, with the borohydride ion serving as the reducing agent.
One of the advantages of using this compound over other reducing agents is that it is more stable and less reactive, which can allow for greater control over the reaction conditions and selectivity. However, it is also more expensive and less widely available than some other reducing agents.
Application
Sodium triacetoxyborohydride is a reducing agent used in organic chemistry for the reduction of various functional groups such as carbonyl groups, oximes, imines, and nitro groups.
It is often used as a milder and more selective alternative to other reducing agents such as sodium borohydride or lithium aluminum hydride for reducing carbonyl compounds (aldehydes and ketones) to their corresponding alcohols. It is also useful for reducing imines and nitriles to amines, as well as for the reduction of other functional groups such as epoxides and azides.