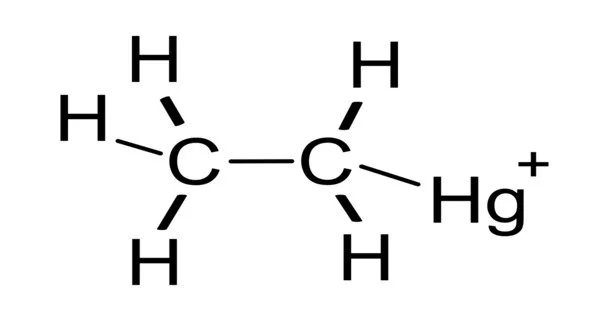
Ethylmercury is a type of organometallic cation with the chemical formula C2H5Hg+. The organomercury compound ethylmercury is made up of an ethyl group bonded to a mercury atom. It is composed of an organic CH3CH2- species (an ethyl group) bound to a mercury(II) centre. It is also known by its chemical name, thiomersal or ethylmercury chloride. Thimerosal is the primary source of ethylmercury. Certain vaccines and pharmaceutical products contain ethylmercury as a preservative.
Thiomersal, also known as ethylmercury chloride, contains 49% mercury by weight. To prevent bacterial and fungal contamination, it has been used as a preservative in some vaccines and other medical products. It works especially well in multidose vials, which use the same container to administer multiple doses.
Synthesis and structure
Ethylmercury (C2H5Hg+) is a compound substituent: it is found in compounds with the formula C2H5HgX, where X is a chloride, thiolate, or another organic group. The most well-known example is X = the mercaptide group of thiosalicylic acid, as in thiomersal. Ethylmercury is most commonly encountered in the body as derivatives with a thiolate attached to the mercury. Hg(II) has a linear or sometimes trigonal coordination geometry in these compounds. Because mercury and carbon have comparable electronegativities, the mercury-carbon bond is described as covalent.
Ethylmercury is distinct from methylmercury, another organomercury compound formed through the methylation of inorganic mercury. Methylmercury is a well-known environmental toxin that can accumulate in fish and other organisms, posing health risks when consumed in large amounts.
Extensive research has been conducted to evaluate the potential health effects of ethylmercury. The World Health Organisation (WHO), the United States Food and Drug Administration (FDA), and the European Medicines Agency (EMA) have all agreed that the use of ethylmercury as a preservative in vaccines poses no significant risk to human health.
Toxicity
The toxicity of ethylmercury has been extensively researched. Ethylmercury, like methylmercury, distributes to all body tissues, crossing the blood-brain barrier and the placental barrier, and it moves freely throughout the body. Extrapolating from methylmercury dose-response relationships, risk assessments for effects on the human nervous system have been made. Estimates suggest that ethylmercury clears from blood in adult humans with a half-life of 3-7 days; however, this area has not been thoroughly studied.
Thiomersal has been phased out or reduced in many childhood vaccines in several countries due to concerns about mercury exposure, particularly in infants and young children. This was done as a precautionary measure, despite the fact that there was no conclusive evidence that the low levels of ethylmercury used in vaccines caused harm.
Public health concerns
Concerns about the effects of methylmercury led to the removal of thimerosal from childhood vaccines in the United States in 1999, but it is still used in all multi-dose vaccines and flu shots (though many single-use vaccines without thimerosal are available).
Researchers have argued that risk assessments based on methylmercury were overly conservative, given that ethylmercury is eliminated from the body and brain much faster than methylmercury. Furthermore, the same researchers have argued that, despite having a much longer half-life in the brain, inorganic mercury metabolised from ethylmercury is far less toxic than inorganic mercury produced from mercury vapour, for unknown reasons.