Sodium oxide is a white solid that is formed when sodium is burned in the absence of oxygen or when sodium peroxide or sodium hydroxide is reduced with the required amount of sodium. It’s a chemical compound with the formula Na2O that’s commonly found in ceramics and glasses. When sodium is burned in the air, a strong, white light is produced. It burns much brighter when exposed to oxygen. It reacts violently with water to form sodium hydroxide, and it reacts violently with acids to form salt solutions. Cubic crystals of sodium monoxide type. It forms a mixture of sodamide and sodium hydroxide as it dissolves in liquid ammonia.
Sodium ions react with oxygen in soil and rocks in the earth’s crust to form sodium oxide (Na2O), one of the most common types of sodium. When water is added to sodium oxide, it produces NaOH, which is the base anhydride of sodium hydroxide.
Na2O + H2O → 2 NaOH
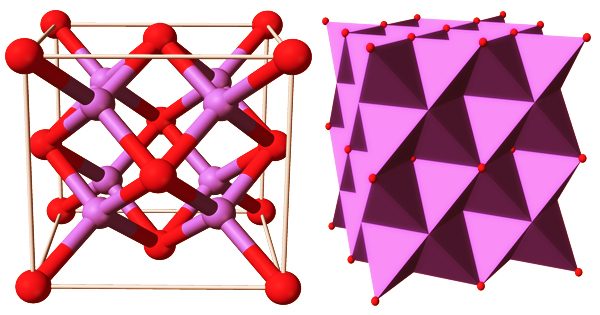
Sodium is a low-toxicity heavy alkaline flux that produces brightly colored glazes. Its exact melting point is 1652°F (900°C), but it starts melting at around 1580°F (860°C), so high-sodium glazes should be reduced at this point. M2O (M = Li, Na, K, Rb) is an alkali metal oxide that crystallizes in an antifluorite structure. The anions and cations’ positions are reversed in this motif compared to CaF2, with sodium ions tetrahedrally coordinated to 4 oxide ions and oxide cubically coordinated to 8 sodium ions.
Two sodium cations and one oxygen anion make up sodium oxide molecules. Na2O molecules have two sodium-oxygen ionic bonds as a result. The reaction of sodium with sodium hydroxide, sodium peroxide, or sodium nitrite produces it.:
2 NaOH + 2 Na → 2 Na2O + H2
Na2O2 + 2 Na → 2 Na2O
2 NaNO2 + 6 Na → 4 Na2O + N2
The majority of these reactions depend on sodium to reduce something, whether it’s hydroxide, peroxide, or nitrite. At all temperatures, sodium oxide is a strong flux. Often the insoluble source of sodium oxide includes some potassium oxide; feldspars, for example, which are the most common source of sodium in high-fire glazes, typically contain varying quantities of sodium and potassium oxides. Burning sodium in air will produce Na2O and about 20% sodium peroxide Na2O2.
6 Na + 2 O2 → 2 Na2O + Na2O2
Sodium oxide is a rigid ceramic that has been ionically bonded. This metal oxide is insoluble in water and will react with it to form sodium hydroxide if exposed to it. Decomposing the sodium salt of ascorbic acid at temperatures above 209o Celsius is a more accessible way to make it in the lab. Since most sodium oxide sources are soluble, they can be used as deflocculants in glaze slurries and casting slips. Glazes with soluble sodium oxide sources like soda ash, borax, or nepheline syenite, such as copper red or carbon trap shinos, can easily become deflocculated.
The fact that sodium oxide is volatile above 2012°E (1100°C) causes flashing on a clay body as it leaves the glaze is an interesting feature. It’s commonly used in vapor glazing techniques like soda and salt firing for this purpose. Most glass contains sodium oxide, though it is added in the form of “soda” (sodium carbonate). In most cases, processed glass contains about 15% sodium oxide, 70% silica (silicon dioxide), and 9% lime (calcium oxide).
As sodium oxide is exposed to water, it is converted to sodium hydroxide, which is extremely corrosive and can cause burns to any human tissue it comes into contact with. As a result, sodium oxide should be treated with caution. The sodium carbonate “soda” acts as a flux, lowering the melting temperature of the silica mixture. Since sodium has a low viscosity and surface tension, it can cause glazes to run if used in large enough quantities. It also has a high rate of expansion and contraction, which causes crazing in glazes. Sodium oxidation produces a soft glaze that is easily scratched by cutlery, acids, and bases (detergents).
Sodium oxide is not soluble in water; when exposed to water, it reacts violently, forming sodium hydroxide. Sodium feldspars, Minspar, Kona F-4, NC-4, Bainbridge, Eureka, Godfrey, and cryolite are all insoluble sources of sodium oxide. Custer, G-200, K-200, Plastic Vitrox, Cornwall stone, volcanic ash, and rottenstone are all potash feldspars that contain it. Soda ash, baking soda, salt, borax, unwashed wood, Gerstley borate, and its substitutes, and sodium silicate are all soluble types of sodium oxide. Some frits and nepheline syenite are slightly soluble.
Information Sources: