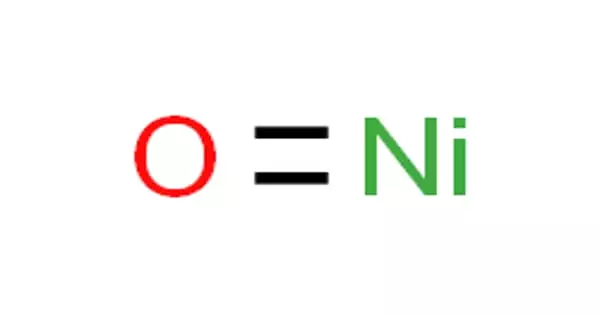
The chemical compound with the formula NiO is nickel(II) oxide. It is the most common nickel oxide. It takes the form of odorless green-black cubic crystals (yellow when heated) or green powder. It’s categorized as a basic metal oxide. Several million kilograms of varying quality are produced annually, primarily as an intermediate in the production of nickel alloys.
It is used in porcelain painting, electroplating, and glass coloring. It is also used in the production of fuel cell electrodes, ferrites (such as NiOFe2O3), nickel salts, nickel catalysts, varistors, and propellants for automotive airbags.
Properties
Green cubic crystals; transforms to a grayish-black octahedral form, known as black oxide, when strongly ignited; the black oxide has a metallic luster; density of green oxide is 6.72 g/cm3; Mohs hardness 5.5.
Bunsenite, a mineralogical form of NiO, is extremely rare. Other nickel(III) oxides, such as Ni2O3 and NiO2, have been claimed, but they have yet to be proven by X-ray crystallography. When nickel oxide is heated with hydrogen, carbon, or carbon monoxide, it is reduced to metallic nickel. At high temperatures (>700 °C), it combines with sodium and potassium oxides to form the corresponding nickelate.
- Molecular Weight: 74.69
- Appearance: Black Powder
- Melting Point: 1955 °C (3551 °F)
- Boiling Point: N/A
- Density: 6.67 g/cm3
- Solubility in H2O: N/A
- Color: green
- Specific Gravity: 6.67
Production
NiO can be made in a variety of ways. When nickel powder is heated above 400 °C, it reacts with oxygen to form NiO. Green nickel oxide is produced in some commercial processes by heating a mixture of nickel powder and water at 1000 °C; the rate of this reaction can be increased by the addition of NiO. Pyrolysis of nickel(II) compounds such as hydroxide, nitrate, and carbonate yields a light green powder, which is the simplest and most successful method of preparation. The synthesis of elements by heating the metal in oxygen can result in grey to black powders, indicating nonstoichiometry.

Structure
NiO has an octahedral Ni2+ and O2– site structure, similar to NaCl. The rock salt structure is a popular name for this conceptually simple structure. NiO, like many other binary metal oxides, is frequently non-stoichiometric, which means the Ni:O ratio deviates from 1:1. This non-stoichiometry in nickel oxide is accompanied by a color change, with stoichiometrically correct NiO being green and non-stoichiometric NiO being black.
Preparation
Pure nickel powder is heated with oxygen at temperatures above 400°C to produce nickel (II) oxide. Green Nickel (II) oxide is produced commercially by heating a mixture of nickel powder and water in the air at 1,000°C. The addition of some Nickel (II) oxide to the above mixture accelerates the reaction. A different method of producing green oxide involves the thermal decomposition of an oxo acid salt of nickel at high temperatures. Thus, heating nickel nitrate, nickel sulfate, or, more conveniently, nickel carbonate to 1,000°C produces green oxide. The black oxide, on the other hand, is created at a lower temperature through incomplete calcination of the carbonate or nitrate salt at 600°C. The oxygen content of the black form is slightly higher than that of the green form.
Reactions
The reactions of nickel oxide with mineral acids produce a number of nickel salts. As a result of the reaction of black nickel oxide with hot dilute sulfuric acid, nickel sulfate, NiSO4•6H2O, is formed. When heated, dilute nitric acid, hydrochloric acid, and hydrobromic acid react with the black form of nickel oxide to produce corresponding nickel salts as hexahydrates.
Heating nickel oxide with hydrogen, carbon, or carbon monoxide reduces it to metallic nickel.
Nickel oxide combines with sodium or potassium hydroxide at elevated temperatures (>700°C), forming sodium or potassium nickelate; i.e., K2NiO2:
NiO + 2NaOH → Na2NiO2 + H2O
Applications
NiO has a wide range of specialized applications, and applications generally distinguish between “chemical grade,” which is a relatively pure material used for specialty applications, and “metallurgical grade,” which is primarily used for alloy production.
In the ceramic industry, it is used to make frits, ferrites, and porcelain glazes. Nickel steel alloys are made from sintered oxide. It was also used in the nickel-iron battery, also known as the Edison Battery, and it is used in fuel cells.
Until the development of the environmentally superior NiMH battery, NiO was used to manufacture the NiCd rechargeable batteries found in many electronic devices. In complementary electrochromic devices, NiO, an anodic electrochromic material, has been widely studied as counter electrodes with tungsten oxide, a cathodic electrochromic material.