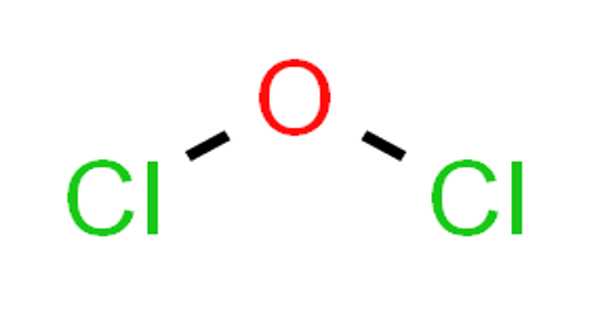
Silver oxide is the chemical compound with the formula Ag2O. It is a dark brown or black powder that is insoluble in water. It is a fine black or dark brown powder that is used to prepare other silver compounds. It is a basic oxide, meaning that it reacts with acids to form salts and water. It is an important compound with a range of useful properties that make it suitable for various applications.
Silver oxide is a dark brown or black solid that is odorless and insoluble in water. It is insoluble in water but soluble in acids such as nitric acid. It is a stable compound and does not decompose at room temperature. It is a strong oxidizing agent and can react violently with reducing agents. It can also react with acids to form silver salts.
Properties
- Chemical formula: Ag2O
- Molar mass: 231.735 g·mol−1
- Appearance: Black/ brown cubic crystals
- Odor: Odorless
- Density: 7.14 g/cm3
- Melting point: 300 °C (572 °F; 573 K) decomposes from ≥200 °C
- Solubility in water: 0.013 g/L (20 °C); 0.053 g/L (80 °C)
- Solubility product (Ksp) of AgOH: 1.52·10−8 (20 °C)
- Solubility: Soluble in acid, alkali; Insoluble in ethanol
- Crystal structure: Cubic
Preparation
Silver oxide can be prepared by combining aqueous solutions of silver nitrate and an alkali hydroxide. This reaction does not afford appreciable amounts of silver hydroxide due to the favorable energetics for the following reaction:
2AgOH⟶ Ag2O + H2O (pK = 2.875)
With the right conditions, this reaction can produce Ag2O powder with properties suitable for a variety of applications, including fine-grained conductive paste filler.
Applications
This oxide is a component of silver-oxide batteries. Silver oxide is used as a mild oxidizing agent in organic chemistry. It oxidizes aldehydes to carboxylic acids, for example. When the silver oxide is prepared in situ from silver nitrate and alkali hydroxide, such reactions often work best.
Silver oxide is often used in silver oxide batteries, which are a type of primary battery that uses silver oxide as the positive electrode and zinc as the negative electrode. It is also used in some chemical reactions as an oxidizing agent, as well as a catalyst in some organic reactions.