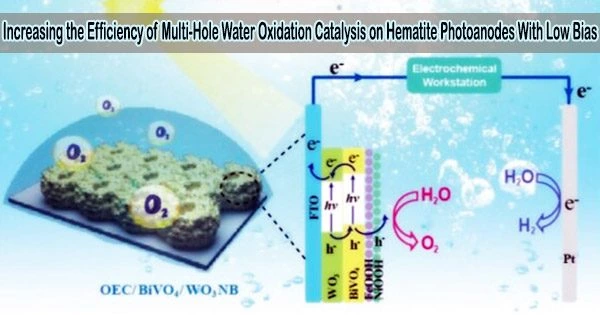
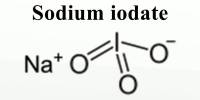
Hematite (α-Fe2O3) is a promising material for photoelectrochemical (PEC) water splitting due to its low cost, abundance, stability, and suitable band gap. Hematite photoanodes have been extensively studied as a means of producing hydrogen gas from water through PEC water splitting.
Because of its usefulness in solar energy conversion, the photoelectrochemical (PEC) water oxidation reaction has received a lot of study in recent years. More and more studies have shown that the accumulation of multiple surface-trapped holes (e.g., high-valent iron oxo on hematite surfaces) or “oxidizing equivalents” is a prerequisite for efficient PEC water oxidation process for such a sluggish four-hole and four-proton transfer reaction in a long timescale of ms-s.
Previous PEC water oxidation studies commonly apply high potentials (>1.2 VRHE) to achieve this key. However, it is unknown how to accomplish multi-hole transfer at low bias (around the onset potential).
A study, published in published in the journal Science China Chemistry and led by Prof. Yuchao Zhang (Key Laboratory of Photochemistry, Chinese Academy of Sciences), sought to address this topic.
Zhang’s research team investigated the significance of excitation energy in the multi-hole accumulation process. UV stimulation greatly increased the buildup of numerous surface-trapped holes, cathodically changing the onset potential by 220 mV and boosting the PEC water oxidation activity by an order of magnitude, according to PEC characterizations and rate law analyses.
Subsequent bulk transport dynamics and surface charge-transfer kinetics revealed that, when compared to visible-light excitation, UV excitation reduced the probability of self-trapped excitons formation and resulted in a ~3 to 5-fold increase in surface holes, which accounted for the promoted multi-hole accumulation process.
Because of these advantages, UV excitation contributes around 40% of total photocurrent under 1 sun illumination, but occupying only 6% of the incident light. This technique could potentially be used to boost other multi-hole catalysis (such as thioether and nitrite oxidation) under low bias.