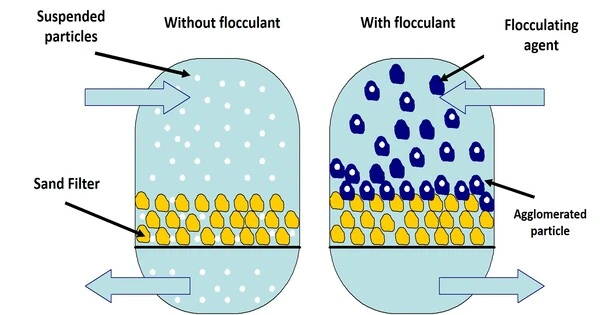
In colloidal chemistry, flocculation is the process by which colloidal particles move from suspension to sediment in the form of floc or flake, either naturally or as a result of the addition of a clarifying agent. It is a process in which colloidal particles combine to create bigger clumps or aggregates known as flocs. Colloidal particles are extremely tiny, ranging from 1 nanometer to 1 micrometer, and frequently carry an electric charge. This charge can cause particles to reject one another and prevent them from settling out of a liquid solution.
Prior to flocculation, colloids are just suspended in the form of a stable dispersion (where the internal phase (solid) is spread throughout the external phase (fluid) through mechanical agitation), rather than truly dissolved in solution. Coagulation and flocculation are significant water treatment processes, with coagulation used to destabilize and aggregate particles by chemical interactions between the coagulant and colloids, and flocculation used to sediment the destabilized particles by forcing them to aggregate into flocs.
Applications
Flocculation is widely employed in a variety of industries, including water treatment, wastewater treatment, and the manufacturing of certain foods and beverages. The purpose of flocculation is to increase the effectiveness of particle removal or separation.
Flocculation is the process of adding a flocculating substance to a suspension. This agent can be a chemical or mechanical device that causes particles to aggregate. The flocculating substance neutralizes the charges on the colloidal particles, allowing them to bind together and form flocs. Once the particles have agglomerated into bigger flocs, they can be easily removed using sedimentation, filtration, or other separation methods.
In water and wastewater treatment, flocculation is an important phase in the purification process. It aids in the removal of suspended particulates, bacteria, and other contaminants, making water safe for consumption or release into the environment. Furthermore, flocculation is used in a variety of industrial processes in which the separation of tiny particles from liquids is critical for product quality and efficiency.