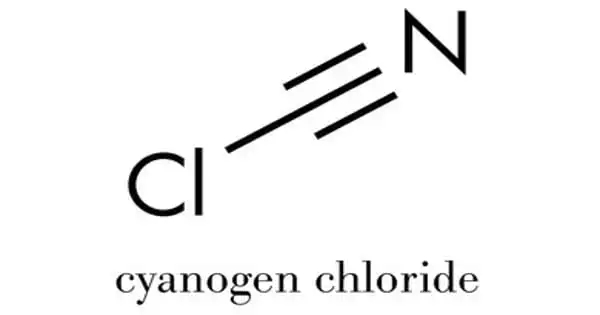Cyanogen chloride, also known as NCCl, is a toxic chemical compound. It is a highly flammable and toxic chemical asphyxiant that impairs the body’s ability to use oxygen. This linear, triatomic pseudohalogen is a colorless gas that can be easily condensed. The related compound cyanogen bromide, a room-temperature solid that is widely used in biochemical analysis and preparation, is more commonly encountered in the laboratory.
Cyanogen chloride is currently used in the manufacture of nylon and synthetic rubber, pigments and dyes, printing and photography, metal cleaning, and metalcore hardening. It is also used in the synthesis of chemicals and in fumigation. Typically, cyanogen chloride is stored and transported as a compressed liquefied gas.
Synthesis, basic properties, structure
CK is a colorless liquid with a low boiling point and, as a result, is very volatile, despite its low water solubility. Its vapors are both irritating and corrosive. The pungent odor, which is highly irritating to the mucous membranes, nearly completely masks the bitter almond aroma and causes excessive tear flow.
The molecule cyanogen chloride has the connectivity ClCN. Carbon and chlorine share a single bond, while carbon and nitrogen share a triple bond. It, like the related cyanogen halides, is a linear molecule (NCF, NCBr, NCI). The oxidation of sodium cyanide with chlorine yields cyanogen chloride. The intermediate cyanogen ((CN)2) is used in this reaction.
NaCN + Cl2 → ClCN + NaCl
The compound trimerizes in the presence of acid to the heterocycle called cyanuric chloride.
Cyanogen chloride is slowly hydrolyzed by water to release cyanate and chloride ions
ClCN + H2O → NCO– + Cl– + 2H+ (at neutral pH)
Although low concentrations of cyanide in raw waters are converted to cyanogen chloride by chlorination, cyanogen chloride may also be formed during the production of chloramines in situ as a residual disinfectant to maintain the distribution system’s hygienic condition. Where chloramination is practiced, treatment must be optimized to minimize the formation of cyanogen chloride while maintaining adequate chloramine residuals.
Applications in synthesis
Cyanogen chloride is a precursor to sulfonyl cyanides and chlorosulfonyl isocyanate, both of which are useful organic reagents. It is an excellent starting material for the synthesis of a wide range of carbonimidic dichlorides. It produces cyano derivatives by reacting with the sodium salts of malonic acid and acetoacetate derivatives.
Uses
Cyanogen chloride is used as a reagent in the synthesis of other compounds, as well as in tear gas and fumigant gases. Cyanogen chloride can be produced as a byproduct of water chloramination or chlorination. It is also formed as a result of the chlorination of the cyanide ion in raw water.
Safety
Cyanogen chloride (CK) poisoning can be fatal in a matter of minutes. It has systemic (whole-body) effects, especially on the organ systems most sensitive to low oxygen levels: the central nervous system (brain), the cardiovascular system (heart and blood vessels), and the pulmonary system (lungs).
Cyanogen chloride is a highly toxic blood agent that was proposed for use in chemical warfare at one point. When it comes into contact with the eyes or respiratory organs, it causes immediate injury. Drowsiness, rhinorrhea (runny nose), sore throat, coughing, confusion, nausea, vomiting, edema, loss of consciousness, convulsions, paralysis, and death are all possible symptoms of exposure. According to US analysts, it is especially dangerous because it is capable of penetrating the filters in gas masks.