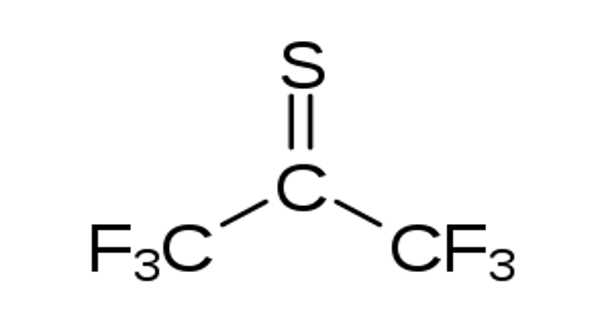
Hexafluorothioacetone is a perfluorothione organic compound with the formula CF3CSCF3. It is a fully fluorinated thioacetone derivative that is used in organic synthesis. It is a blue gas under normal conditions.
Production
Hexafluorothioacetone was first produced by Middleton in 1961 by boiling bis-(perfluoroisopropyl)mercury with sulfur.
Properties
Hexafluorothioacetone boils at 8°C. Below this, it is a blue liquid.
Colour
The blue color is caused by visible light absorption with bands at 800–675 nm and 725–400 nm. T1–S0 and S1–S0 transitions cause these bands. There is also a strong absorption in the ultraviolet region between 230 and 190 nm.
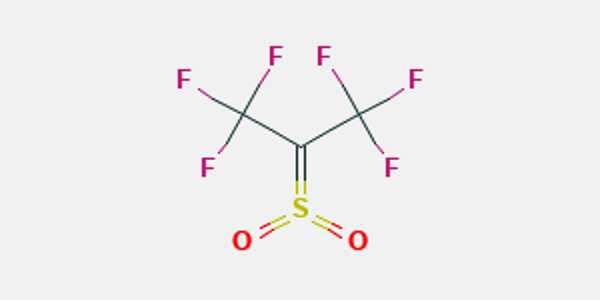
Reactions
Because it cannot form thioenol compounds (=C-S-H) and the sulfur is not in a negative ionized state (C-S), hexafluorothioacetone behaves more like a true thiocarbonyl (C=S) than many other thiocarbonyl compounds. At standard conditions, hexafluorothioacetone is not attacked by water or oxygen, as are many other thiocarbonyls.
Bases cause the dimer 2,2,4,4-tetrakis-(trifluoromethyl)-1,3-dithietane to form. Amines are bases. Heat the dimer to regenerate the hexafluorothioacetone monomer. The dimer is also formed in a reaction involving hexafluoropropene, sulfur, and potassium fluoride.
Hexafluorothioacetone reacts with bisulfite to form a Bunte salt CH(CF3)2SSO2–.
Mercaptans reacting with hexafluorothioacetone yield disulfides or a thiohemiketal:
R-SH + C(CF3)2S → R-S-S-CH(CF3)2.
R-SH + C(CF3)2S → RSC(CF3)2SH (for example in methyl mercaptan or ethyl mercaptan).
Instead of a thiohemiketal, water elimination with mercaptoacetic acid produces a ring-shaped molecule known as a dithiolanone –CH2C(O)SC (CF3) 2,2-di(trifluoromethyl)-1,3-dithiolan-4-one (2S). The formation of a dimeric disulfide CH by aqueous hydrogen chloride (CF3)2SSC(CF3)2Cl. The reaction of hydrogen bromide with water produces a similar result. CH(CF)2SSC(CF3)2Br. Dry hydrogen iodide, on the other hand, reduces the sulfur, resulting in CH(CF3)2SH. Only a disulfide CH is formed when wet hydrogen iodide is reduced (CF3)2SSC(CF3)2H. When strong organic acids react with water, they form the disulfide compound CH(CF3)2SSC(CF3)2OH.