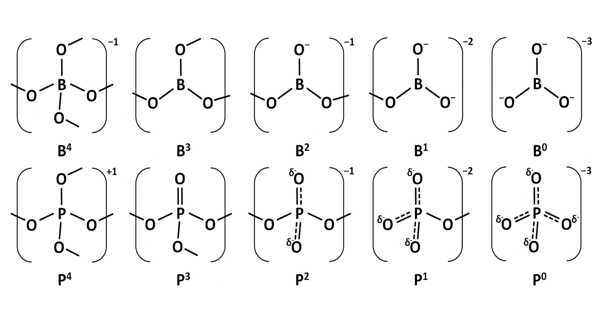
Borophosphates are mixed anion compounds composed of borate and phosphate anions that may be linked by a common oxygen atom. Borophosphates have complex anionic structures made up of BO4, BO3, and PO4 groups, as well as their partially protonated species. Compounds containing water or hydroxy groups can also be classified as compounds.
A first approach to developing borophosphate structural chemistry is based on linking principles of the primary building units that follow the general line of silicate crystal chemistry. Borophosphate crystal structures are first divided into anhydrous and hydrated phases.
Borophosphates are classified according to whether or not they are hydrated, as well as their anion structure, which can be a single, double, triple, isolated ring, isolated branched ring, a simple chain, branched-chain, loop chain, layers, or three-dimensional network. The borate phosphates are single anion compounds with separate borate and phosphate groups. Some borophosphate structures are silicate-like.
Related compounds include aluminophosphates, which contain aluminum instead of boron, gallophosphates, which contain gallium instead of boron, and boroarsenates, boroantimonates, and vanadoborate by substituting the phosphate.
Formation
Borophosphates can be formed by combining compounds at temperatures of up to 900 °C. The products are dense and anhydrous, with no organic substances. To dissolve the product, a non-water solvent such as ethylene glycol is used in solvothermal synthesis. At around 171, the flux method crystallizes from a molten flux of boric acid and sodium dihydrogen phosphate.
The hydrothermal method heats the ingredients under pressure with water up to 200 °C. Boric acid, phosphoric acid, metal salts, or organic bases are among the ingredients. Hydrogen is frequently found in products. As a solvent in the ionothermal synthesis method, an ionic liquid such as 1-alkyl-3-methylimidazolium bromide is used. This can be done at atmospheric pressure and temperatures under 100 °C.
Characteristics
Magnetic, electrical, optical, and catalytic properties of borophosphate compounds have been investigated. Because some borophosphates are porous, there is a surface for interaction on their interiors as well as their surface. They can absorb water reversibly or have channels that allow ions to conduct. Because a labeled tetrahedron’s reflection cannot be superimposed (even with rotation or movement), compounds containing phosphate and borate tetrahedrons can be non-centrosymmetric, or chiral.