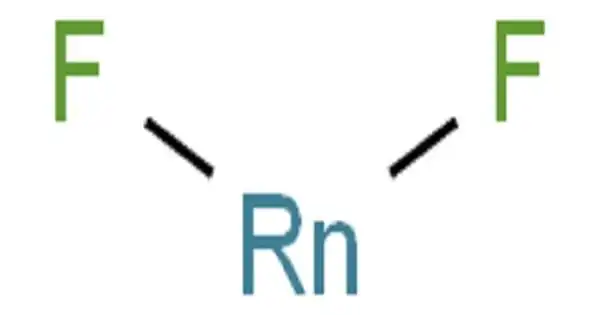
Radon difluoride (RnF2) is a compound chemical. It is a radioactive noble gas. Radon rapidly interacts with fluorine to produce a solid compound, but when vaporized, it decomposes and the specific composition is unknown. Calculations indicate that, unlike all other known binary noble gas compounds, it may be ionic. The radioactivity of radon limits the effectiveness of radon compounds. The longest-living isotope, radon-222, has a half-life of only 3.82 days before decaying through -emission to produce polonium-218.
Radon (Rn) is a chemical element that is produced by the radioactive decay of radium. It is a heavy radioactive gas in Periodic Group 18 (noble gases). Radon is a colorless gas that weighs 7.5 times more than air and more than 100 times more than hydrogen.
Preparation
When radon is heated to 400 °C with fluorine, radon difluoride is formed.
Reactions
Radon difluoride can be reduced to radon and hydrogen fluoride when heated with hydrogen gas at 500 °C.
Properties and Occurrences
Radon has a melting point of -71°C, a boiling temperature of -61.8°C, a gas density of 9.73 g/l, a liquid specific gravity of 4.4 at -62°C, and a solid specific gravity of 4, generally with a valence of 0. At normal temperatures, radon is a colorless gas. It is also the most dense of the gases. It emits a beautiful phosphorescence when chilled below its freezing point. As the temperature is reduced, the phosphorescence turns yellow, then orange-red at the temperature of liquid air.
Radon is a rare element in nature since its isotopes are all short-lived and its source, radium, is a scarce element. Radon traces can be found in the atmosphere near the ground due to seepage from soil and rocks, both of which contain trace amounts of radium.
Radon atoms have an unusually stable electrical structure of eight electrons in the outer shell, which explains the element’s chemical inactivity. Radon, on the other hand, is not inert chemically. The existence of radon difluoride, for example, which appears to be more chemically stable than compounds of the two reactive noble gases, krypton and xenon, was discovered in 1962. The short lifetime of radon and its high-energy radioactivity make it challenging to investigate radon compounds experimentally.