Organometallic Chemistry
Definition
Organometallic (or OM) Chemistry is the study of compounds containing, and reactions involving, metal-carbon bonds. Organometallic Chemistry combines aspects of inorganic chemistry and organic chemistry. Living systems contain a variety of organometallic compounds, prominent examples being hemoglobin and chlorophyll. The specialized field focused on the study of such compounds is known as bioinorganic chemistry. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.
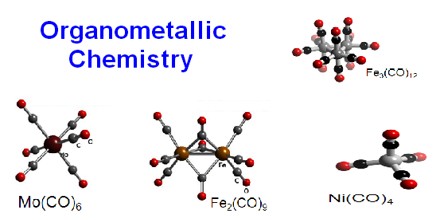
As in other areas of chemistry, electron counting is useful for organizing organometallic chemistry. The 18-electron rule is helpful in predicting the stabilities of metal carbonyls and related compounds. Most organometallic compounds do not however follow the 18e rule. Chemical bonding and reactivity in organometallic compounds is often discussed from the perspective of the isolobal principle.
As well as X-ray diffraction, NMR and infrared spectroscopy are common techniques used to determine structure. The dynamic properties of organometallic compounds are often probed with variable-temperature NMR and chemical kinetics.
Organometallic Compounds are widely used both stoichiometrically in research and industrial chemical reactions, as well as in the role of catalysts to increase the rates of such reactions, as in uses of homogeneous catalysis, where target molecules include polymers, pharmaceuticals, and many other types of practical products.
Organometallic Compounds
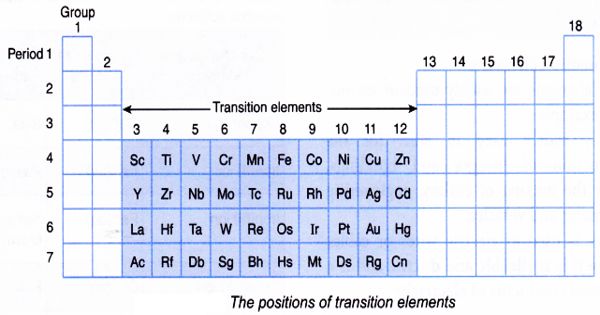
Organometallic Compounds are often distinguished by the prefix “organo-,” as in organopalladium compounds. They are also known as organo-inorganics, metallo-organics, and metalorganics. Examples of such organometallic compounds include all Gilman reagents that contain lithium and copper, and Grignard reagents that contain magnesium. Tetracarbonyl nickel and ferrocene are examples of organometallic compounds containing transition metals.
The term “metalorganics” usually refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands.[citation needed] Metal beta-diketonates, alkoxides, and dialkylamides are representative members of this class.
In addition to the traditional metals, lanthanides, actinides, and semimetals, elements such as boron, silicon, arsenic, and selenium are considered to form organometallic compounds, e.g. organoborane compounds such as triethylborane (Et3B).
Biological systems contain a variety of organometallic compounds. Examples include hemoglobin and myoglobin, each of which contains an iron center bonded to a porphyrin ring; and chlorophyll, with magnesium at the center of a chlorin ring.

Applications of Organometallic Compounds
Organometallic Compounds find practical use in stoichiometric and catalytically active compounds. Tetraethyl lead previously was combined with gasoline as an antiknock agent. Due to the toxicity of lead, it is no longer used, and it has been replaced by other organometallic compounds such as ferrocene and methylcyclopentadienyl manganese tricarbonyl (MMT). The Monsanto process utilizes a rhodium-carbonyl complex to manufacture acetic acid from methanol and carbon monoxide industrially. The Ziegler-Natta catalyst is a titanium-based organometallic compound used in the production of polyethylene and other polymers.
In the production of fine chemicals and pharmaceuticals, Ryoji Noyori’s chiral ruthenium-BINAP complex catalytically reduces beta-ketoesters to secondary alcohols.
Structure and Properties
In the absence of direct structural evidence for a carbon–metal bond, some compounds are not considered to be organometallic. Thus, the status of compounds in which the canonical anion has a delocalized structure in which the negative charge is shared with an atom more electronegative than carbon may vary with the nature of the anionic moiety, the metal ion, and possibly the medium.
Depending mostly on the nature of the metallic ion and somewhat on the nature of the organic compound, the character of the bond may either be ionic or covalent. Organic compounds bonded to sodium or potassium are primarily ionic. Those bonded to lead, tin, or mercury, are considered as having covalent bonds, and those bonded to magnesium or lithium have bonds with intermediate properties.
Organometallic Compounds with bonds that have characters in between ionic and covalent are very important in industry, as they are both relatively stable in solution and relatively ionic to undergo reactions. Two important classes are organolithium compounds and Grignard reagents. In certain organometallic compounds, such as ferrocene or dibenzenechromium, the pi orbitals of the organic moiety are believed to be involved in binding to the metal.