Tetrahymena ribozyme refers to a catalytic RNA molecule found in the single-celled organism Tetrahymena thermophila. Ribozymes are RNA molecules that can catalyze chemical reactions, similar to the way enzymes function in proteins.
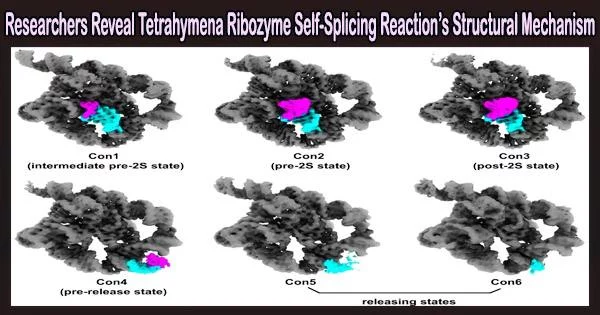
The mechanism of group 1 introns coordinating self-splicing events was revealed when a team led by Prof. Zhang Kaiming from the University of Science and Technology (USTC) used cryogenic electron microscopy (cryo-EM) to solve six conformations in the second-step self-splicing of Tetrahymena ribozyme. Their work was published in Nature Communications.
Group Ⅰ introns are catalytic RNAs that can fold into tertiary structure with a active site with the help of metal ions, thereby promoting catalysis without proteins. However, because RNA is flexible and heterogeneous, analyzing RNA structures is extremely difficult.
Numerous research conducted since the discovery of Tetrahymena group I introns have clarified the underlying catalytic mechanism, however the complete 3D structure of the molecule and its rearrangements have not yet been determined.
The Tetrahymena ribozyme was first discovered in the 1980s by researchers Sidney Altman and Thomas Cech, who received the Nobel Prize in Chemistry in 1989 for their work on catalytic RNA. It was the first time that RNA was found to have enzymatic activity, challenging the long-held belief that only proteins could catalyze reactions.
To resolve the structural mechanism and the conformation changes in the self-splicing reactions, Prof. Zhang Kaiming’s team first designed the substrates for the self-splicing reactions.
The scientists used single-particle cryo-EM analysis to take structural snapshots of various conformations of Tetrahymena ribozyme during the self-spicing process after confirming that the substrates could carry out the catalytic reactions and figuring out the ideal reaction conditions.
To create the P1-P1 extension duplex, the oligonucleotide substrate base pairs with the apoenzyme in the first step. Tertiary contacts between P1 and three single-stranded regions in the catalytic core then mediate the docking of the duplex into the active site.
Then the oligonucleotide product of the first step is released. The 3′-exon substrates are docked in the ribozyme during the second phase, the ligation reaction, through tertiary contacts and metal coordination. The reaction then gradually transitions to an intermediate stage, during which the metal coordination deteriorates and the tertiary contacts disappear.
The exons are ligated in this period. Sequentially, the ribozyme relaxed and the product duplex undocks, reaching the substrate-free site. Finally, the duplex is unpaired and the product is released.
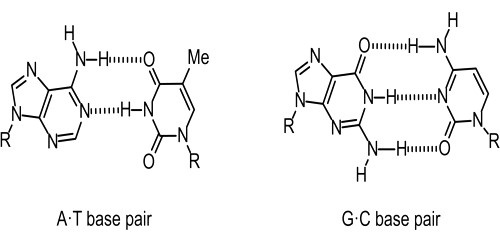
Additionally, the researchers discovered structural proof of some hydrogen bonds and metal ions being rearranged on active sites, which supports catalysis and controls self-splicing events.
This study demonstrated the benefits of employing cryo-EM to clarify RNA structure by revealing the atomic-level workings of the self-splicing reaction. As cryo-EM is used more and more in the study of structurally diverse molecules, it will become an essential tool in numerous other biological studies, such as the folding of RNA systems.