The inorganic compound sodium nitride has the chemical formula Na3N. It has the appearance of a yellowish-white crystalline solid. Sodium nitride, unlike lithium nitride and some other nitrides, is an extremely unstable alkali metal nitride. It can be created by combining sodium and nitrogen atomic beams deposited on a low-temperature sapphire substrate. It easily decomposes into its constituent parts –
2 Na3N → 6 Na + N2
Synthesis
Sodium nitride can be produced in two ways: thermal decomposition of NaNH2 or direct reaction of the elements. Dieter Fischer and Martin Jansen, as well as Grigori Vajenine, used the latter method to successfully synthesize sodium nitride. The first method involves separately introducing desired Na and N2 ratios in the gas phase and depositing them in a vacuum chamber on a cooled substrate, which is then heated to room temperature (298 K) to crystallize.
The second method is to use a metal surface to react elemental sodium with plasma-activated nitrogen. This synthesis can be aided further by adding liquid Na-K alloy to the compound, then removing the excess liquid and washing with fresh alloy. A centrifuge is then used to separate the solids from the liquid. Vajenine’s method, on the other hand, is very air-sensitive and can decompose and combust quickly unless exposed to a pure oxygen (O2) environment.
Characteristics
Because of intrinsic properties, sodium nitride can be reddish-brown or dark blue in color depending on how it is synthesized. After several weeks at room temperature, it shows no signs of decomposition. The compound has no melting point because it decomposes back into its elemental forms around 360 K, as demonstrated by mass spectrometry. The compound’s estimated enthalpy of formation is +64 kJ/mol.
Structure
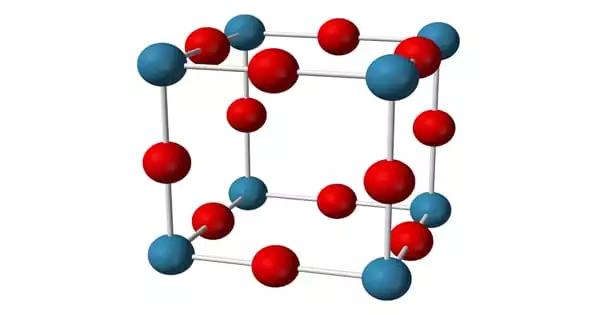
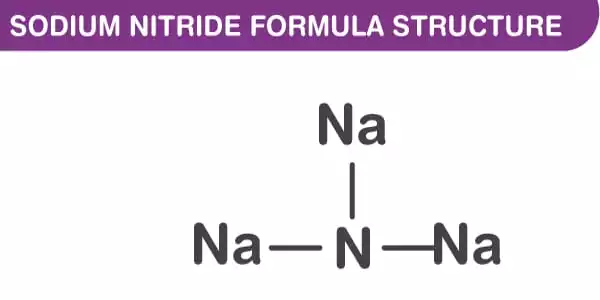
The anti-ReO structure can be seen in the sodium nitride formula. At room temperature, it appears to be about 90% ionic, but it has the bandgap typical of a semiconductor. Furthermore, sodium nitride is made up of a simple lattice of NNa Octaherdra. It has an anti-ReO3 structure and a simple lattice of NNa6 octahedra. The NNa bond lengths in the compound are 236.6 pm. The N-Na bond has a length of approximately 236.6 pm. This structure has been confirmed using powder and single-crystal X-ray diffraction and, more recently, neutron diffraction.
The X-ray diffraction method can be used to record the structural formation of sodium nitride. The anion of sodium amide and sodium imide is the same as that of sodium nitride. Similarly, Lithium nitride and Potassium nitride have the same cation.
Uses
Sodium nitrite is an inorganic sodium salt with the counterion nitrite. It is used as a food preservative and as an antidote to cyanide poisoning. It functions as an antimicrobial food preservative, an antihypertensive agent, a food antioxidant, a poison, and a cyanide antidote. It is a nitrite salt as well as an inorganic sodium salt.