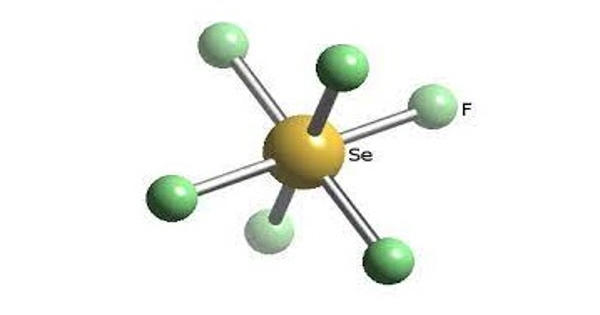
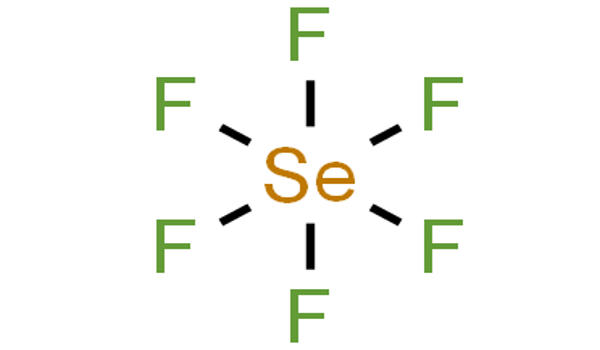
Selenium hexafluoride is a colorless gas used as a electrical insulator. It is the inorganic compound with the formula SeF6. It is very toxic colourless gas described as having a “repulsive” odor. When heated to high temperatures it may decompose to emit toxic fluoride and selenium fumes. It is not widely encountered and has no commercial applications. It is only slightly soluble in water. It does not occur naturally in the environment.
Properties
Selenium hexafluoride is a colorless gas. It is much more reactive and toxic than sulfur hexafluoride. It has a bad odor. It is only slightly soluble in water. It does not occur naturally in the environment.
- Melting point: -39°C
- Boiling point: -34,5°C
- Density: 1.66
- Form: colorless gas
- Water Solubility: insoluble H2O
- Critical temperature: 72
- Solubility in water: Insoluble
Structure, preparation, and reactions
Selenium hexafluoride is made by heating selenium and fluorine together. Like many compounds of selenium, SeF6 is hypervalent. The compound has octahedral molecular geometry with an Se−F bond length of 168.8 pm. When released to air, selenium hexafluoride will react with moisture, forming other compounds which are removed from the atmosphere by rainfall.
It is slightly soluble in water. It reacts slowly with water to form other compounds. It may also evaporate from water. SeF6 can be prepared from the elements or by the reaction of bromine trifluoride (BrF3) with selenium dioxide. The crude product is purified by sublimation.
Selenium hexafluoride is prepared by passing fluorine gas over finely divided selenium in a copper vessel: Se + 3F2 → SeF6.
The relative reactivity of the hexafluorides of S, Se, and Te follows the order TeF6 > SeF6 > SF6, the latter being completely inert toward hydrolysis until high temperatures. SeF6 also resists hydrolysis. The gas can be passed through 10% NaOH or KOH without change, but reacts with gaseous ammonia at 200°C.
Uses
Selenium hexafluoride is used as a gaseous electric insulator. It may be prepared by passing gaseous fluorine over finely divided selenium in a copper vessel. Industrial selenium exposure is common for makers of arc light electrodes, electric rectifiers, and semiconductors. It is not widely encountered and has no commercial applications.
Safety
Although selenium hexafluoride is quite inert and slow to hydrolyze, it is toxic even at low concentrations, especially by longer exposure. In the U.S., OSHA and ACGIH standards for selenium hexafluoride exposure is an upper limit of 0.05 ppm in air averaged over an eight-hour work shift. Additionally, selenium hexafluoride is designated as IDLH chemical with a maximum allowed exposure limit of 2 ppm.
Informaton Source: