Radicals play an astounding range of roles in an astounding range of sciences and technologies. Our understanding of their structures’ near-limitless flexibility and the vast scope of their activities has grown tremendously in the last few years.
Scientists at the DOE’s Brookhaven National Laboratory in the United States assisted in measuring how unpaired electrons in atoms at one end of a molecule can drive chemical reactivity on the molecule’s opposite side. This work, done in collaboration with Princeton University, is described in a paper recently published in the Journal of the American Chemical Society as showing how molecules containing these so-called free radicals could be used in a whole new class of reactions.
“Most free radical reactions take place at the site of the unpaired electron,” explained Brookhaven Lab chemist Matthew Bird, one of the paper’s co-corresponding authors. The Princeton group had become experts in the use of free radicals in a variety of synthetic applications, including polymer upcycling. However, they have speculated that free radicals may influence reactivity on other parts of the molecule as well, by pulling electrons away from those more distant locations.
“Our measurements show that these radicals can have strong ‘electron-withdrawing’ effects that make other parts of the molecule more reactive,” Bird explained.
The Princeton team demonstrated how that long-distance pull can overcome energy barriers and bring together otherwise unreactive molecules, potentially leading to a new approach to organic molecule synthesis.
Most free radical reactions take place at the site of the unpaired electron. Our measurements show that these radicals can have strong ‘electron-withdrawing’ effects that make other parts of the molecule more reactive.
Matthew Bird
Combining capabilities
The study drew on the resources of a Princeton-led DOE Energy Frontier Research Center (EFRC) focusing on Bio-Inspired Light Escalated Chemistry (BioLEC). The collaboration brings together top synthetic chemists and groups with cutting-edge spectroscopic techniques for studying reactions.
“This project is an example of how BioLEC’s combined expertise enabled the team to quantify an important physical property of these radical species, which in turn allowed us to design the resulting synthetic methodology,” said Robert Knowles, who led Princeton’s role in this research.
The Brookhaven team’s major contribution is a technique known as pulse radiolysis, which is only available at Brookhaven and one other location in the United States.
“We use the Laser Electron Accelerator Facility (LEAF)—part of the Accelerator Center for Energy Research (ACER) in Brookhaven’s Chemistry Division—to generate intense high-energy electron pulses,” Bird explained. “These pulses allow us to add or subtract electrons from molecules to make reactive species that might be difficult to make using other techniques, including short-lived reaction intermediates. With this technique, we can step into one part of a reaction and monitor what happens.”
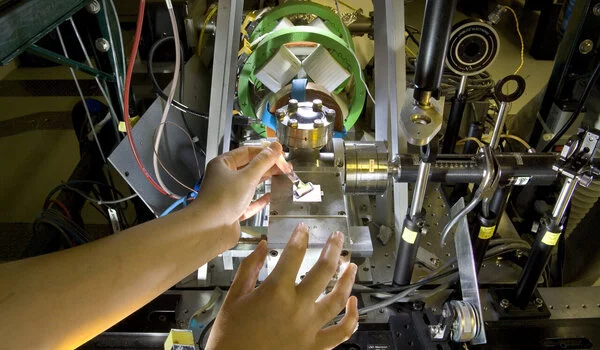
For the current study, the team used pulse radiolysis to generate molecules with oxygen-centered radicals, and then measured the “electron-withdrawing” effects on the other side of the molecule. They measured the electron pull by tracking how much the oxygen at the opposite side attracts protons, positively charged ions sloshing around in solution. The stronger the pull from the radical, the more acidic the solution has to be for protons to bind to the molecule, Bird explained.
The Brookhaven scientists found the acidity had to be high to enable proton capture, meaning the oxygen radical was a very strong electron withdrawing group. That was good news for the Princeton team. They then demonstrated that it’s possible to exploit the “electron-withdrawing” effect of oxygen radicals by making parts of molecules that are generally inert more chemically reactive.
“The oxygen radical causes a transient’polarity reversal’ within the molecule, causing electrons that would normally prefer to stay on the distant side to move toward the radical, making the ‘far’ side more reactive,” Bird explained.
These discoveries enabled the development of a novel substitution reaction on phenol-based starting materials to produce more complex phenol products.
“This is an excellent example of how our pulse radiolysis technique can be applied to cutting-edge scientific problems,” Bird said. “We were delighted to host an outstanding graduate student from the Knowles group, Nick Shin, for this collaboration. In this second phase of BioLEC, we are looking forward to more collaborative projects and seeing what new problems we can solve using pulse radiolysis.”