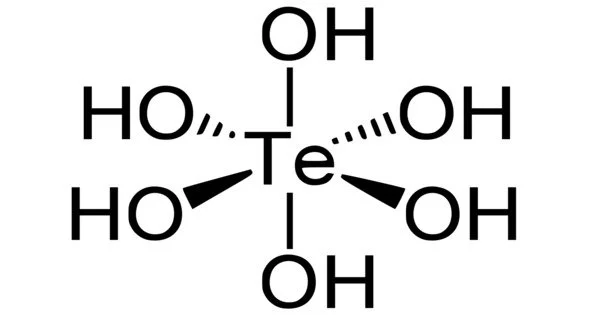
Telluric acid is a chemical compound with the formula Te(OH)6. It is a very weak acid that is a good oxidizing agent and can be obtained in two crystalline forms and as a tetrahydrate by oxidizing tellurium or tellurium dioxide. It is a white solid composed of octahedral Te(OH)6 molecules that persist in aqueous solution. There are two types: rhombohedral and monoclinic, and both contain octahedral Te(OH)6 molecules.
Telluric acid is a dibasic weak acid that forms tellurate salts with strong bases and hydrogen tellurate salts with weaker bases or upon hydrolysis of tellurates in water. It is used as a tellurium source in the synthesis of oxidation catalysts.
Properties
- Chemical formula: Te(OH)6
- Molar mass: 229.64 g/mol
- Appearance: White monoclinic crystals
- Density: 3.07 g/cm3
- Melting point: 136 °C (277 °F; 409 K)
- Solubility in water: 50.1 g/100 ml at 30 °C
- Conjugate base: Tellurate
- Molecular shape: octahedral

Preparation
Telluric acid is produced by oxidizing tellurium or tellurium dioxide with a strong oxidant such as hydrogen peroxide, chromium trioxide, or sodium peroxide. Tellurium salts are formed by fusing tellurium with potassium nitrate plus carbonate, or by the oxidizing action of chlorine on a tellurite in alkaline solution.
TeO2 + H2O2 + 2 H2O → Te(OH)6
Crystallization of telluric acid solutions below 10 °C gives Te(OH)6·4H2O. It is oxidizing, as shown by the electrode potential for the reaction below, although it is kinetically slow in its oxidations.
H6TeO6 + 2 H+ + 2 e– ⇌ TeO2 + 4 H2O, Eo = +1.02 V
Chlorine, by comparison, is +1.36 V and selenous acid is +0.74 V in oxidizing conditions.
Reactions
The anhydrous acid is stable in air at 100 °C but above this it dehydrates to form polymetatelluric acid, a white hygroscopic powder (approximate composition [H2TeO4)10], and allotelluric acid, an acid syrup of unknown structure (approximate composition [H2TeO4)3(H2O)4].
Typical salts of the acid contains the anions [Te(O)(OH)5]– and [Te(O)2(OH)4]2-. The presence of the tellurate ion TeO42- has been confirmed in the solid state structure of Rb6[TeO5][TeO4]. Strong heating at over 300 °C produces the α crystalline modification of tellurium trioxide, α-TeO3. Reaction with diazomethane gives the hexamethyl ester, Te(OCH3)6.
Telluric acid and its salts mostly contain hexacoordinate tellurium. This is true even for salts such as magnesium tellurate, MgTeO4, which is isostructural with magnesium molybdate and contains TeO6 octahedra.