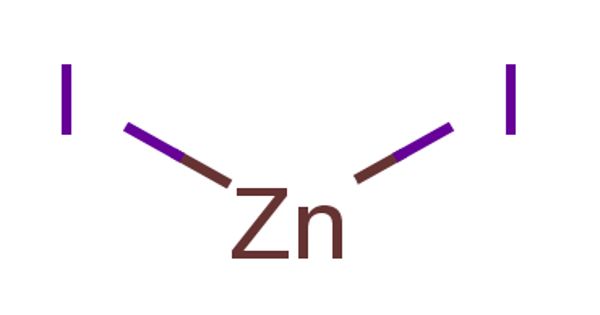
Zinc iodide is the inorganic compound with the formula ZnI2. It is a white solid that readily absorbs water from the atmosphere. It exists both in anhydrous form and as a dihydrate. Both are white and readily absorb water from the atmosphere. It can be manufactured directly by the reaction of zinc and iodine while refluxing ether. It has no major application. It has been used as an antiseptic and astringent.
Preparation
It can be prepared by the direct reaction of zinc and iodine in water or in refluxing ether, or by treating zinc with iodine in aqueous solution:
Zn + I2 → ZnI2
This experiment can be used to illustrate the differences between metallic and non-metallic elements and their reaction to form a compound – a metal salt – with new properties.
The reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed I2, and electrolysis.
Zn(s) + I2(s) → No Reaction
Zn(s) + I2(s) H2O→ ZnI2(aq)
Zn2+ + 2I- → Zn + I2
The reaction can be easily reversed using electrolysis to decompose the compound back into its elements. These are easily recognizable from their distinctive appearances.

Properties
Zinc is a gray metal which is used in the form of small granules in this experiment. Solid iodine is a black, shiny solid, but is not a metal according to chemical classifications. The structure of solid ZnI2 is unusual relative to the dichloride. While zinc centers are tetrahedrally coordinated, as in ZnCl2, groups of four of these tetrahedra share three vertices to form “super-tetrahedra” of composition {Zn4I10}, which are linked by their vertices to form a three-dimensional structure. These “super-tetrahedra” are similar to the P4O10 structure.
- Molecular Weight: 319.18
- Appearance: White Powder
- Melting Point: 446° C (834.8° F)
- Boiling Point: 1150 °C (2102 °F)
- Density: 4.74 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 317.738083
Applications
- Zinc iodide is often used as an x-ray opaque penetrant in industrial radiography to improve the contrast between the damage and intact composite.
- It used as a stain in electron microscopy
- In combination with osmium tetroxide, ZnI2 is used as a stain in electron microscopy.
- It used as an X-ray opaque penetrant in industrial radiography
- As a Lewis acid, zinc iodide catalyzes for the conversion of methanol to triptane and hexamethylbenzene.
- It used as a catalyst in the conversion of methanol to triptane
Information Source: