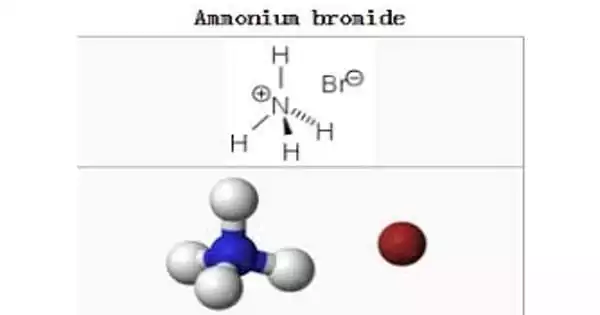
Ammonium bromide, NH4Br, is the ammonium salt of hydrobromic acid. It appears as white, odorless crystals or granules that turn yellow when exposed to air. The chemical crystallizes in colorless prisms with a saline flavor; it sublimes on heating and is freely soluble in water. It eventually becomes yellow when exposed to air due to the oxidation of residues of bromide (Br–) to bromine (Br2).
Ammonium bromide is an ammonium salt comprised of ammonium and bromide ions in a 1:1 ratio. It is an ammonium salt and a bromide salt. The ammonium bromide formula remains stable under the suggested storage circumstances, which have a temperature chosen appropriate to the ammonium bromide’s need.
Properties
Ammonium Bromide is odourless white crystals or granules which becomes yellow when exposed with air. It is the ammonium salt of hydrobromic acid. The chemical matures in prisms that are colorless, owning a salty taste. It sublimes when heated and is very easily soluble in the water.
- Molecular weight: 97.943 g/mol
- Density: 2.429 g/cm3
- Crystal structure: Isometric
- Boiling point: 452 °C
- Melting point: 235 °C
- Appearance: Colorless to white crystals or powder
- Solubility in H2O: Soluble.

Preparation
Ammonium bromide can be formed by the process of a direct action of the hydrogen bromide on the ammonia. The preparation of the Ammonium bromide can also be done by the process of reacting to the ammonia with iron (II) bromide or iron (III) bromide, which we can attain by passing the aqueous bromine solution over the iron filings.
Ammonium bromide can be prepared by the direct action of hydrogen bromide on ammonia.
NH3 + HBr → NH4Br
It can also be prepared by the reaction of ammonia with iron(II) bromide or iron(III) bromide, which may be obtained by passing aqueous bromine solution over iron filings.
2 NH3 + FeBr2 + 2 H2O → 2 NH4Br + Fe(OH)2
Reactions
Ammonium bromide is a weak acid with a pKa of ~5 in water. It is an acid salt because the ammonium ion hydrolyzes slightly in water.
Ammonium Bromide is strong electrolyte when put in water:
NH4Br(s) → NH4+(aq) + Br−(aq)
Ammonium bromide decomposes to ammonia and hydrogen bromide when heated at elevated temperatures:
NH4Br → NH3 + HBr
Uses
Ammonium bromide is employed in photography in films, plates, and papers, as well as in wood fireproofing, lithography and process engraving, corrosion inhibitors, and pharmaceutical preparations. It is also employed in conjunction with an appropriate oxidizer, and it is an effective biocide that is used to manage bacteria, fungi, and algae in various water systems in the industrial sector and wastewater.
- It is used in photography.
- It is used in fireproofing wood.
- It is used as a corrosive inhibitor.