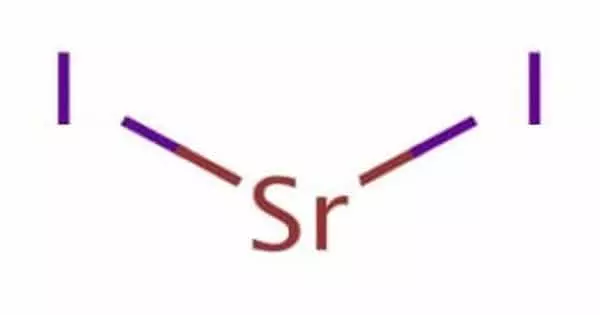
Strontium iodide (SrI2) is a strontium and iodine salt. It is an ionic, water-soluble, and deliquescent compound that can be used as a potassium iodide substitute in medicine. Due to its optical clarity, relatively high density, high effective atomic number (Z=48), and high scintillation light yield, it is also used as a scintillation gamma radiation detector, typically doped with europium. The toxicity of strontium compounds is of the lowest order. Calcium is chemically and biologically similar to it.
Because it is a water-soluble, deliquescent compound, it absorbs moisture from the atmosphere and undergoes a minor physical change. When heated to high temperatures in the presence of air, it produces strontium oxide and iodine.
Properties
It appears as a colorless or white crystalline solid. It is odorless. The mass of one mole of strontium iodide is 341.43 g/mol. The compound melts at a temperature of 507-645 °C (780-918 K, 945-1193 °F). It boils at a temperature of 1773 °C (2046 K, 3223 °F). The density of its anhydrous form is 4.55 g/cm3, and its hexahydrate form is 4.40 g/cm3. It is highly soluble in water at room temperature and sparingly soluble in ethanol, the solubility being 177 g/100 ml at 20 °C and 3.1 g/100 ml at 4 °C respectively.
- Molecular Weight: 341.43
- Appearance: White Crystalline Solid
- Form: Powder
- Melting point: 515°
- Boiling Point: 1,773° C (3,223° F)
- Density: 4.550
- Storage & Sensitivity: Hygroscopic, Light Sensitive, Ambient temperatures.
- Solubility: Soluble in water and ethanol.

Reactions
Strontium iodide can be prepared by reacting strontium carbonate with hydroiodic acid:
SrCO3 + 2 HI → SrI2 + H2O + CO2
When exposed to air, strontium iodide transforms from a white powder to a yellowish powder. Strontium iodide completely decomposes at high temperatures (in the presence of air) to form strontium oxide and free iodine.
Production
In recent years, europium-doped strontium iodide (SrI2:Eu2+) has emerged as a promising scintillation material for gamma-ray spectroscopy, outperforming the widely used high-performance commercial scintillator LaBr3:Ce3+ in light yield and proportional response. Several companies are commercializing large-diameter SrI2 crystals grown reliably using the vertical Bridgman technique.
Applications
As a scintillation gamma radiation detector, strontium iodide is used. It’s also used in a variety of handheld radiation detectors, as well as medical, industrial, and environmental applications. It is used in medicine to replace potassium iodide. Europium-doped SrI2 is used as a scintillator for detecting gamma radiation due to its high density, atomic number, scintillation light yield, and optical clarity.