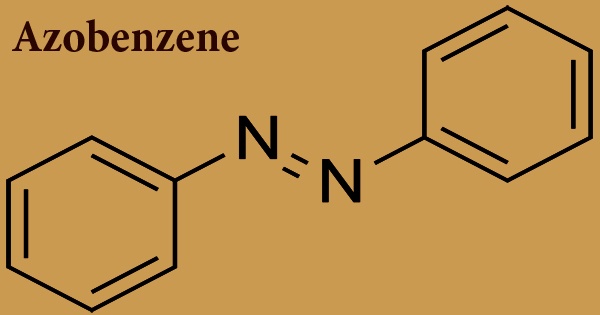
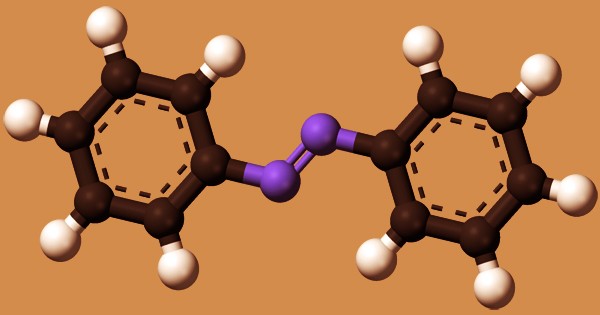
Azobenzene is a molecule whose structure consists of two phenyl rings bound by a double bond of N=N; the azobenzene class of compounds’ parent compound. It is the simplest instance of a compound of aryl azo. To refer to a broad class of related compounds, the word ‘azobenzene’ or simply ‘azo’ is sometimes used. Azobenzene occurs as crystals of orange-red or dark brown chunky solids. These azo compounds are known as diazene (diimide) derivatives and are often referred to as ‘diazenes’. The diazenes strongly absorb light and are traditional dyes.
Azobenzene is sensitive to air and light; air may create an explosive mixture of dust; water is insoluble. Eilhard Mitscherlich first mentioned it in 1834. In 1856, yellowish-red crystalline flakes were obtained from azobenzene. Its initial form is equivalent to the current one. Nitrobenzene is reduced by iron filings in the presence of acetic acid, according to the 1856 process. This applies in particular to organic azides which, through the addition of metal salts or strong acids, have been sensitized. Acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents form toxic gases by combining the materials in this class.
Bovine serum albumin binds to azobenzene. Secondary valences are part of the interaction between azobenzene and other azo compounds with proteins. In modern synthesis, in the presence of a base, zinc is the reductant. It also employs industrial electrosynthesis using nitrobenzene. The trans isomer is about 50 kJ/mol more stable, and about 100 kJ/mol is the limit to isomerization in the ground state. By mixing materials with alkali metals in this category, flammable gases are produced. With strong oxidizing agents, metal salts, peroxides, and sulphides, explosive combinations can occur.
Azobenzene is a weak base but undergoes protonation with a pKa = -2.95 at one nitrogen. It acts as a foundation of Lewis, e.g. against the trihalides of boron. The ordinary azobenzene in the trans-form is almost everything. On exposure to light, it is partially converted into the cis-form. Azobenzene (and derivatives) are subjected to trans and cis isomer photoisomerization. Cis-Azobenzene relaxes the trans isomer back into darkness. Such thermal relaxation is sluggish at room temperature. This chemical is incompatible with oxidizing agents that are solid.
Factors influencing the in vitro metabolism of azo compounds have been studied and conditions have been described that allow optimum metabolism. The wavelengths at which azobenzene isomerization takes place depend on each azo molecule’s precise structure, but they are generally grouped into three classes: azobenzene-type molecules, aminoazobenzene-type molecules, and pseudo-stilbenes. Due to the slight variations in their electronic absorption spectrums, these azos are yellow, orange, and red, respectively. Under acidic conditions in the stomach, azobenzene can be converted to the recognized human carcinogen benzidine non-enzymatically. The mechanism of isomerization has been the subject of some debate, with two pathways defined as viable: N-N bond rotation, double bond disruption, or semi-linear and hybridized transition state rotation through inversion. With a dry chemical, carbon dioxide or Halon extinguisher, fires involving this substance may be managed. The photo-isomerization of azobenzene is a type of molecular motion induced by light. On larger scales of time, this isomerization can also lead to motion. For example, in random places, polarized light can cause the molecules to isomerize and relax.
Information Sources: