Carbocatalysis is a “green” option for catalytic transformations in the gas phase as well as in the liquid phase. It is a form of catalysis that uses heterogeneous carbon materials for the transformation or synthesis of organic or inorganic substrates. The astounding physicochemical properties of carbon materials earned them a well-deserved ubiquitous presence in the industry. The catalysts are characterized by their high surface areas, surface functionality, and large, aromatic basal planes. Carbons with a large surface area such as active carbons and carbon black powders have since ever been considered as supports to deposit transition metals that were the relevant catalytic centers. Carbocatalysis can be distinguishable from supported catalysis (such as palladium on carbon) in that no metal is present, or if metals are present they are not the active species.
As of 2010, the mechanisms of reactivity are not well understood. However, the important field of catalysis is still dominated by transition metals even though carbon potential as catalyst was probed more than 3 decades ago.
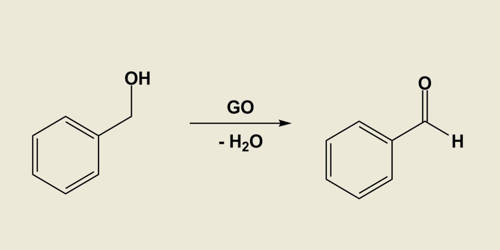
Carbocatalysis for liquid‐phase reactions, especially for organic synthesis, is an emerging research discipline and has undergone rapid development in recent years. One of the most common examples of carbocatalysis is the oxidative dehydrogenation of ethylbenzene to styrene discovered in the 1970s. Also in the industrial process of (non-oxidative) dehydrogenation of ethylbenzene, the potassium-promoted iron oxide catalyst is coated with a carbon layer as the active phase. In another early example, a variety of substituted nitrobenzenes were reduced to the corresponding aniline using hydrazine and graphite as the catalyst. It is one green option for catalytic transformation in the gas phase as well as in the liquid phase.
The discovery of nanostructured carbon allotropes such as carbon nanotubes, fullerenes, or graphene promoted further developments. This is evident by the numerous reports on gas‐phase dehydrogenation and selective oxidation where carbon can be used as a successful alternative to metal oxide systems. Oxidized carbon nanotubes were used to dehydrogenate n-butane to 1-butene, and to selectively oxidize acrolein to acrylic acid. Fullerenes were used in the catalytic reduction of nitrobenzene to aniline in the presence of H2.