Boron (B) is a non-metallic element of group 13 (IIIa, or boron group) and is the only non-metallic element of the periodic table of elements. With the symbol B and the atomic number 5, it is a chemical element. It is a low-abundance element in the Solar System and in the Earth’s crust, formed entirely by cosmic ray spallation and supernovae and not by stellar nucleosynthesis. It is a non-metallic trivalent component that occurs abundantly in borax and ulexite evaporite ores. Boron has the formal state of oxidation III in the most familiar compounds. Oxides, sulfides, nitrides, and halides comprise these. Boron is electron-deficient, with an empty p-orbital. It has many types, in which amorphous boron, a dark powder, unresponsive to oxygen, water, acids, and alkalis are the most common. It interacts to form borides with metals. Boron is a poor electrical conductor at normal temperatures but is a decent conductor at elevated temperatures.
Sir Humphry Davy and J.L. Gay-Lussac discovered ‘Boron’ in 1808. In 1892, Henri Moissan isolated a purer form of boron. Eventually, by sparking a mixture of boron chloride, BCl3 vapor, and hydrogen, E. Weintraub in the USA produced totally pure boron. Boron was found to have very different properties to those previously recorded in the material so collected. Boron comprises about 0.001 percent of the Earth’s crust by weight. The water-solubility of its more popular naturally occurring compounds, the borate minerals, is based on Earth. These, such as borax and kernite, are industrially mined as evaporites. In Turkey, the largest producer of boron minerals, the largest known boron deposits are found.
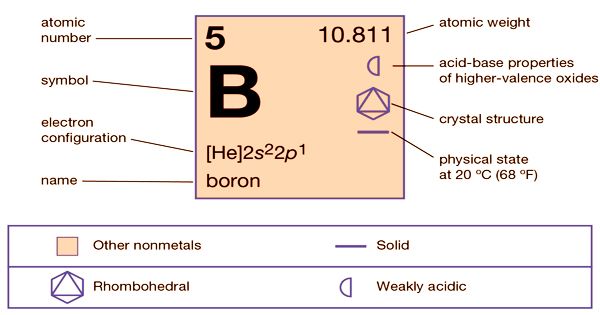
Boron (B)
Boron is a very strong, black substance with a high melting point; it is present in many polymorphs; it occurs as charcoal-grey parts or black powder or as crystalline. It has many types, and amorphous boron, a dark powder, is the most common, non-reactive to oxygen, water, acids, and alkalis. It interacts to form borides with metals. Boron is a micronutrient important for plants. Boron, at high temperatures, conducts electricity like metal and is almost an insulator at low temperatures. Some abrasives, such as carborundum, are difficult enough (9.3 on the Mohs scale) to scrape, but too fragile for tool use. It represents about 0.001 percent of the Earth’s crust by weight.
By contrast with carbon, which boron resembles chemically, the term boron was coined from borax, the mineral from which it was isolated. Boron occurs in combination with borax, kernite, and tincalconite (hydrated sodium borate), the main commercial minerals of boron, especially concentrated in the arid regions of California, and minerals such as colemanite, ulexite, and tourmaline, which are widely distributed. Natural boric acid from Sassolite occurs in Italy in particular. Boron compounds are used in organic synthesis, in the manufacture of glasses of a specific form, and as wood preservatives. For advanced aerospace structures, because of their high strength and lightweight, boron filaments are used.
Boron occurs in some volcanic spring waters as orthoboric acid, and as borates in the borax and colemanite of the stone. Extensive deposits of borax are present in Turkey. Rasorite is by far the most powerful source of boron, however. It is found in California, USA, in the Mojave Desert. By reduction of its bromide or chloride (BBr3, BCl3) with hydrogen on an electrically heated tantalum filament, pure crystalline boron may be prepared with difficulty. By reducing boron trichloride, or tribromide with hydrogen, on electrically heated filaments, high-purity boron is prepared. By heating the trioxide with magnesium powder, it can prepare impure, or amorphous, boron.
In its outer shell, Boron has only three electrons, which makes it more metal than non-metal. In their Valence shell, nonmetals have four or more electrons. Even so, in period 2, boron is somewhat linked to metalloids and also to non-metals. Restricted amounts of elemental boron are commonly used in steel to improve hardness. It is present in many steels, typically in the range of 0,001 to 0,005 percent, added as the iron alloy ferroboron. As a rocket fuel igniter, and in pyrotechnic flares, amorphous boron is used. It gives a distinctive green color to the flares.

Boron Chunks
Boric (or boracic) acid, borax (sodium borate), and boric oxide are the most significant compounds of boron. These can be used in eye drops, mild antiseptics, powder cleaning, and glazing of tiles. Borax used to be used as a food preservative and to make bleach. It has a melting point of 2,079 °C, a boiling point of 2,550 °C, and a density of 2.37 g/cm3. Boric oxide is also widely used in borosilicate glass processing (Pyrex). It makes glass durable and resistant to fire. Borosilcate glass is made from fibreglass textiles and insulation.
A total of 13 boron isotopes exist, two of which are stable. As contained in the Earth’s crust, the stable-isotope B-10 provides 19.85 percent of the element’s abundance, and the isotope B-11 provides 80.2 percent of the Earth’s boron abundance. By reducing volatile boron halides with hydrogen at high temperatures, pure boron can be prepared. Boron compound processing does not require elemental boron formation but takes advantage of the convenient availability of borates. In the non-ferrous-metals industry, boron is often used, typically as a deoxidizer, as a degasifier in copper-based alloys and high-conductance copper, and in aluminum castings to process the grain.
For the cell walls of plants, Boron is important. It is not known to be harmful to animals, but it can affect the metabolism of the body at higher doses. As a potential cure for brain tumors, such boron compounds are being tested. Tiny, carefully controlled quantities of boron are applied as a doping agent to silicon and germanium in the semiconductor industry to alter electrical conductivity. When humans eat large quantities of food containing boron, the concentrations of boron in their bodies can increase to levels that can cause health problems. Boron can infect the stomach, liver, brain, and kidneys and can inevitably lead to death. Nose, throat, or eye irritation can occur when exposure to small amounts of boron occurs. To render a person sick, it takes 5 g of borc acid and 20 grams or more to place his or her life in danger.
Boron nitride has excellent properties and can be used to produce a diamond-hard material. The nitride also acts like an electrical insulator but, like a metal, conducts heat. Similar to graphite, it also has lubricating properties. Boron occurs naturally by weathering in the atmosphere since it is released into air, soil, and water. In very limited quantities, it can also occur in groundwater. By making glass, combusting coal, melting copper, and applying agricultural fertilizers, human beings add boron. Boron concentrations introduced by humans are lower than the concentrations added naturally by natural weathering.
Elemental boron and borate are not known to be harmful and do not need special health care. Some of the more rare compounds of boron hydrogen, however, are undoubtedly toxic and do need treatment. Most’ boron’ studies include samples containing small quantities of carbon. Boron’s chemical activity resembles that of silicon rather than that of aluminum. Stunted, malformed development are common symptoms of long-term boron deficiency; vegetable “brown heart” and sugar beet “dry rot” are among the disorders due to boron deficiency. Air and drinking water exposure to boron is not very likely to occur, although there is a chance of exposure to borate dust at the workplace. Boron toxicity from consumer goods such as cosmetics and laundry products can also occur.
Information Sources: