Researchers from the Laboratory of Catalysis at the University of Liège (Belgium), Lionel Delaude and François Mazars, have demonstrated that caffeine and theophylline may be used to ‘green’ catalysts based on ruthenium in their research. This chemical element belongs to the transition metals.
The findings of this investigation have been published in the scholarly journal Organometallics and will be available for free use for a period of six months.
Because they allow reactions to be carried out more quickly and selectively under kinder experimental circumstances, catalysts are often used in chemical processes. They are typically made from non-renewable raw materials, frequently extracted from metallurgy and petrochemicals.
Ruthenium is a chemical element with the symbol “Ru” and atomic number 44. It is a transition metal that belongs to the platinum group of elements, which also includes platinum, palladium, osmium, iridium, and rhodium. Ruthenium is a rare and relatively expensive metal.
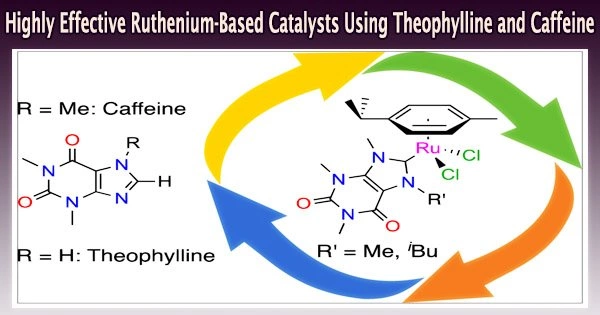
Chemists are currently attempting to minimize the carbon footprint of their procedures in accordance with the twelve “green chemistry” principles. In this context, the team from the Laboratory of Organometallic Chemistry and Homogeneous Catalysis at the University of Liège has developed biobased catalysts derived from caffeine and theophylline, two natural substances from the xanthine family found in large quantities in coffee beans, tea leaves, and cocoa beans.
“The whole process of adding value to these compounds is environmentally friendly, since their extraction and separation from renewable plant sources requires only water and supercritical CO2,” explains Lionel Delaude, professor of chemistry and director of the laboratory. What’s more, they are under-exploited and available at low cost.
Slight modifications to the chemical structure of xanthines can be used to synthesize N-heterocyclic carbene precursors, which form the basis of many catalysts in the 21st century.
The work of Lionel Delaude and his doctoral student François Mazars has shown that the combination of a para-cymene ligand derived from a-phellandrene (an essential oil found in many plants, including dill and eucalyptus) and an N-heterocyclic carbene ligand derived from caffeine or theophylline with ruthenium (a low-toxicity metal from the iron family) leads to the formation of highly effective catalysts for promoting three major types of organic reaction, namely, the transfer hydrogenation of unsaturated substrates with isopropanol, the oxidation of alkenes with sodium periodate, and the synthesis of vinyl esters from 1-hexyne and benzoic acid.
This discovery aids in the advancement of ecologically friendly and sustainable organometallic chemistry. It also opens the door to further catalytic applications that produce bio-sourced N-heterocyclic carbenes by combining caffeine and theophylline.
This publication in Organometallics has been singled out by the editors of the American Chemical Society to be highlighted on the day of its publication (28th May 2023) and to remain in open access for six months as part of the ACS Editors’ Choice programme. It has also been selected to appear on the cover of an issue of the journal (July 10, 2023).