Manganese(VII) oxide, often known as manganese heptoxide, is an inorganic chemical having the formula Mn2O7. It is a dark green explosive oil formed by the action of concentrated sulfuric acid on permanganates. Manganese is at an oxidation state of +7. This is the only manganese compound with a +7 oxidation state that is not a permanganate. It comes in either a dark crimson or green color. This flammable liquid is extremely reactive. It is a hazardous oxidant that was discovered in 1860. It is permanganic acid’s acid anhydride.
Properties
This chemical compound’s crystalline form is dark green. Reflected light turns the liquid green, whereas transmitted light turns it red. It dissolves in carbon tetrachloride and decomposes when exposed to water.
- Appearance: dark red oil (room temp.), green if in contact with sulfuric acid
- Density: 2.79 g/cm3
- Melting point: 5.9 °C (42.6 °F; 279.0 K)
- Boiling point: explodes on heating
- Crystal structure: monoclinic
- Coordination geometry: bitetrahedra

Preparation
Sulfuric acid and potassium permanganate are combined to create it. This reaction results in the dark green oil. When burst, it produces manganese dioxide and oxygen. This reaction could result in the formation of ozone, a potent oxidizing agent. It can be detonated by striking it or interacting with organic substances like alcohol.
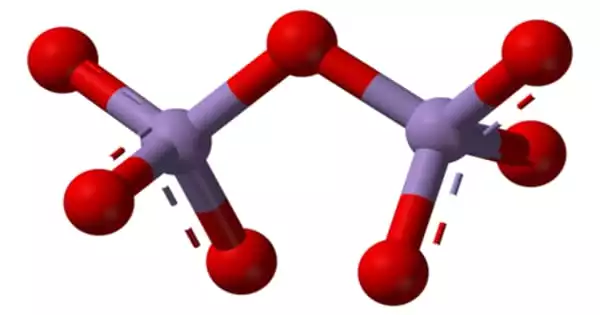
Structure
Its structure confirms that it is a nonpolar molecular species based on its solubility qualities. The molecules are made up of two tetrahedra that share a vertex. The vertices are occupied by oxygen atoms, and the Mn(VII) centers are located at the tetrahedra’s centers. The formula O3Mn−O−MnO3 represents the connection. The terminal M−nO distances are 1.585Å and 1.77Å, respectively, from the two Mn atoms. The angle formed by Mn−O−Mn is 120.7°.
Pyrosulfate, pyrophosphate, and dichromate have structures that are comparable to Mn2O7. Cl2O7 is most likely the most comparable main group species. In terms of transition metal series comparisons, Tc2O7 and Mn2O7 are structurally comparable, however the Tc−O−Tc angle is 180°. Solid Re2O7 is not molecular, but is made up of crosslinked Re centers with both tetrahedral and octahedral sites; in the vapour phase, it is molecular and has a structure similar to Tc2O7.
Toxicity
Manganese heptoxide is a highly reactive oxidant derived from sulfuric acid (H2SO4) and potassium permanganate (KMnO4). It interacts aggressively with practically all organic materials. At all times, proper care must be exercised.