Sodium aluminate is a natural inorganic chemical used in the commercial industry; NaAlO2, NaAl(OH)4 (hydrated), Na2O.Al2O3, or Na2Al2O4 are all names for a white crystalline solid with the formula NaAlO2. It is insoluble in ethanol but soluble in water, resulting in highly alkaline solutions with a melting point of 1800°C. It’s a white crystalline powder in a water solution that’s corrosive to metals and tissue. Many industrial and technological applications rely on it as a reliable source of aluminium hydroxide. Sodium aluminate is available as a solution or a solid in the commercial industry.
Sodium aluminate is made by heating bauxite with sodium carbonate and then removing the residue with water, or by adding excess aluminum to hot condensed sodium hydroxide in the laboratory. In solution the ion Al(OH)4– predominates. Sodium aluminate is used as a mordant, to make zeolites, to treat effluent, to make glass, and to make cleaning compounds. Other sodium aluminate-related compounds include Na5AlO4, which contains distinct AlO45- anions, Na7Al3O8, and Na17Al5O16, which contain complex polymeric anions, and NaAl11O17, which was once incorrectly thought to be β-alumina, a phase of aluminium oxide.
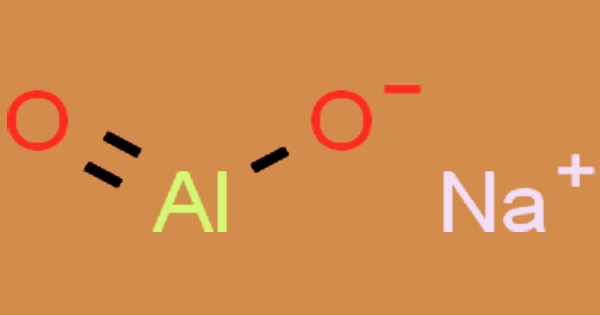
The versatility of sodium aluminate’s technical applications accounts for its commercial value. It is used in water treatment systems as an additive to water softening systems, as a coagulant to remove suspended solids and certain metals (Cr, Ba, Cu), and as a coagulant to remove dissolved silica. Sodium aluminate is used in construction to speed up the solidification of concrete, particularly when working in cold weather. In water, sodium aluminate forms a solid base, reacts violently with acids, and corrodes metals. Copper, tin, zinc, aluminum, acids, arsenic, and chlorocarbons are incompatible.
There have been several methods for making solid sodium aluminate produced. In most processes, the first step is to make an aqueous sodium aluminate solution. The solid phase is then formed by drying the sodium aluminate solution. The dissolution of aluminum hydroxides in a caustic soda solution is a popular method for producing aqueous sodium aluminate. The three-dimensional structure of corner linked AlO4 tetrahedra in anhydrous sodium aluminate, NaAlO2, is made up of corner linked AlO4 tetrahedra. Layers of AlO4 tetrahedra are joined into rings in the hydrated form NaAlO25/4H2O, and the layers are kept together by sodium ions and water molecules that hydrogen bind to O atoms in the AlO4 tetrahedra.
As sodium aluminate dissolves in water, it forms a corrosive alkaline solution. Aluminum hydroxide suspension with excess NaOH is produced. The sodium aluminate solution is then spray dried after the suspension has been passed through heated reaction tubes. At a temperature above the melting point of caustic soda but below 600°C, sodium aluminate is made by mixing sodium hydroxide and subdivided aluminum hydrate in a solid state reaction. It has been stated that sodium aluminate can be extracted from sodium dawsonite, which is contained in oil shales. A semi-solid product is generated by using more concentrated NaOH solutions. The procedure must be carried out in nickel or steel steam-heated vessels, with the aluminium hydroxide being boiled with around 50% aqueous caustic soda until a pulp forms.
Sodium aluminate is used in construction to speed up the solidification of concrete, particularly when working in the cold. It’s also used in the paper industry, as well as in the manufacture of fire bricks and alumina. Intermediates in the processing of zeolites are sodium aluminate solutions. A series of 100 mL Erlenmeyer flasks were filled with 1.5 g of basic aluminum sulfate to research the formation of sodium dawsonite. Then, in each flask, a different amount of 1 M Na2CO3 solution (1, 2, 3, 4,….17 mL) was added.
Water was used to dilute of solution to 25 mL. For 72 hours, the flasks were kept at room temperature. Following this period, the pH of each solution was calculated and the solution was filtered under reduced pressure. Until examination, the solids were dried for 18 hours at 80°C. The action of sodium hydroxide on elemental aluminum, which is an amphoteric metal, also produces sodium aluminate. Once formed, the reaction is highly exothermic and is followed by the rapid evolution of hydrogen gas. The reaction is sometimes written as:
2Al + 2NaOH + 2H2O → 2NaAlO2 + 3H2
Several one-gram samples of sodium dawsonite were heated at various temperatures in the range of 500-1100°C for 30 minutes to determine the thermal decomposition process and the crystallization temperature of sodium aluminate. The solids were characterized using X-ray diffractometry (XRD) and Fourier transform infrared (FTIR) after they were heated (FTIR). It is used in water treatment as a supplement to water softening systems, as a coagulant aid to increase flocculation and to remove dissolved silica and phosphates.
Information Sources: