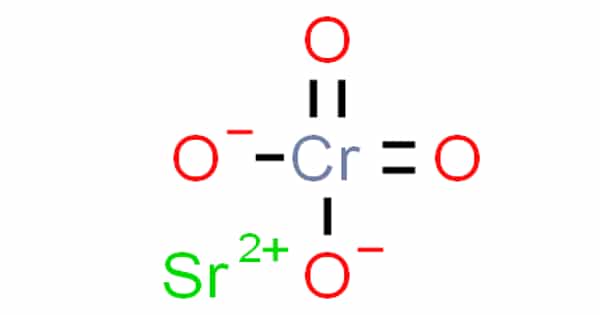
Strontium chromate, also known as SrCrO4, is a chemical compound with the formula SrCrO4. It comes in the form of a light yellow powder or granular solid. It’s not soluble in water. The most serious danger is the threat to the environment. Immediate action should be taken to limit its environmental spread. It is used as a pigment, a corrosion-resistant coating, and in pyrotechnics.
Properties
Strontium Chromate is a yellowish, crystalline inorganic compound that, when heated, emits toxic chromium fumes. Strontium chromate is a strong oxidizing agent and is highly corrosive. It has a high degree of opacity and is resistant to light and heat. Strontium chromate, on the other hand, was not used in watercolors due to its high hiding power.
- Density: 3.89
- Molecular Weight: mol. wt. = 203.62
- Appearance: Yellow powder
- Density: 3.09
- Melting Point: N/A
- Boiling Point: N/A
- Form: Powder
- Specific Gravity: 3.895

Preparation
Strontium chromate is synthesized by reacting strontium chloride with sodium chromate or strontium carbonate with sodium dichromate.
The reaction of strontium chloride with sodium chromate or strontium carbonate with sodium dichromate produces strontium chromate. Because strontium chromate corrosion inhibitors are highly toxic and carcinogenic, their use generates waste streams, poses environmental risks, and raises disposal concerns.
A common corrosion inhibitor is strontium chromate. It has excellent corrosion resistance for metal substrates, especially aluminum substrates. It’s also used as a pigment, a protective coating, and in fireworks. It can be used as an anti-corrosive primer for zinc, magnesium, aluminum, and alloys used in aircraft construction.
Uses
This substance is used as a colorant in polyvinyl chloride, in pyrotechnics, in chrome plating, in aluminum flake coatings, and as a corrosion inhibitor and metal conditioner.
- Corrosion inhibitor in pigments
- In electrochemical processes to control sulphate concentration of solutions
- Colorant in polyvinyl chloride resins
- Pyrotechnics
- Aluminum flake coatings
- As an anti-corrosive primer for zinc, magnesium, aluminum, and alloys used in aircraft manufacture.
Health hazards
Strontium chromate is primarily toxic to the lungs, causing shortness of breath, bronchitis, pneumonia, and asthma, but it can also harm the gastrointestinal tract, liver, kidneys, and immune system. This substance is a known human carcinogen and has been linked to an increased risk of developing lung cancer and sinonasal cavity cancer.