The molecular configuration of a molecule is the permanent geometry that results from the spatial arrangement of its bonds. Configuration, in chemistry, the spatial arrangement of atoms in a molecule. It is an arrangement of parts or elements in a particular form, figure, or combination. The ability of the same set of atoms to form two or more molecules with different configurations is stereoisomerism. The configuration is usually depicted by means of a three-dimensional model (a ball-and-stick model), a perspective drawing, or a plane projection diagram. Used as drugs, compounds with different configurations normally have different physiological activities, including the desired pharmacological effect, the toxicology, and metabolism. Today, optical and chemical methods make it possible to determine the absolute configuration of practically any molecule. On the contrary, Configuration is permanent geometry. It refers to the spatial arrangement of bonds and can be changed only by breaking bonds. E.g., L&D and R&S configuration
The configuration is distinct from chemical conformation, a shape attainable by bond rotations. The configuration is the relative position of the atoms in a molecule that can be changed exclusively by cleaving and forming new chemical bonds. It is the fixed arrangement of atoms dictated by the bonds of a molecule. It means like the fixed three-dimensional relationship of the atoms in a molecule, defined by the bonds between them.
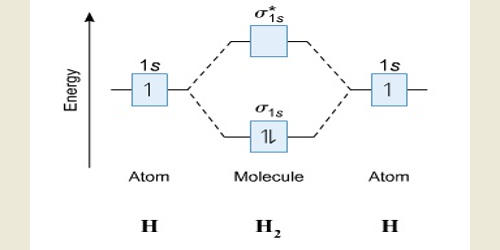
Until late in the 20th century, the experimental determination of absolute or actual configuration (i.e., the true three-dimensional form of the molecule) was a difficult process; therefore, there were few substances with known absolute configurations (e.g., tartaric acid). The three-dimensional shape or configuration of a molecule is an important characteristic. This shape is dependent on the preferred spatial orientation of covalent bonds to atoms having two or more bonding partners. Three-dimensional configurations are best viewed with the aid of models. Molecular orbitals are obtained by combining the atomic orbitals on the atoms in the molecule. Consider the H2 molecule, for example. Electrons are added to molecular orbitals, one at a time, starting with the lowest energy molecular orbital.