Phosphoric Acid
Definition
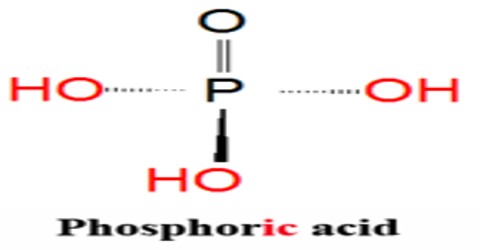
Phosphoric Acid is a clear liquid, or a solid that forms colorless, rhombus-shaped crystals, that is used in fertilizers, detergents, food flavoring, and pharmaceuticals. Chemical formula: H 3 PO 4. It is also used in dental cements, in the preparation of albumin derivatives, and in the sugar and textile industries.
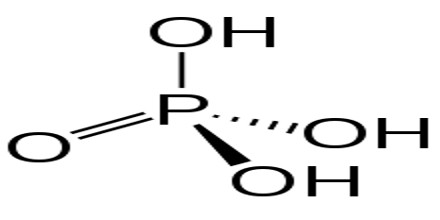
The most common source of phosphoric acid is an 85% aqueous solution; such solutions are colourless, odourless, and non-volatile. The 85% solution is a syrupy liquid, but still pourable. Although phosphoric acid does not meet the strict definition of a strong acid, the 85% solution is acidic enough to be corrosive. Because of the high percentage of phosphoric acid in this reagent, at least some of the orthophosphoric acid is condensed into polyphosphoric acids. For the sake of labeling and simplicity, the 85% represents H3PO4 as if it were all orthophosphoric acid. Dilute aqueous solutions of phosphoric acid exist in the ortho- form.
Phosphoric acid forms three classes of salts corresponding to replacement of one, two, or three hydrogen atoms. Among the important phosphate salts are: sodium dihydrogen phosphate (NaH2PO4), used for control of hydrogen ion concentration (acidity) of solutions; disodium hydrogen phosphate (Na2HPO4), used in water treatment as a precipitant for highly charged metal cations; trisodium phosphate (Na3PO4), used in soaps and detergents; calcium dihydrogen phosphate or calcium superphosphate (Ca[H2PO4]2), a major fertilizer ingredient; calcium monohydrogen phosphate (CaHPO4), used as a conditioning agent for salts and sugars.

Uses of Phosphoric Acid
Phosphoric acid is used as an additive and flavoring agent in both human and animal feed. It is commonly used in sodas to provide a sharp or sour flavor. In fact, almost all the acidic flavor in soda comes from phosphoric acid, as the carbonic acid contained in the bubbles has little effect on the overall pH. Phosphoric acid also helps to keep bacteria and fungi from forming in these sugary drinks. The primary use of phosphoric acid is in the manufacture of fertilizers, although it is also used to make synthetic detergents. Other uses of phosphoric acid include the treatment of water and metal, and it is also sometimes used as an additive in some foods and drinks.
Among many applications, phosphoric acid is used:
- As a solution for anodizing.
- As an external standard for phosphorus-31 nuclear magnetic resonance (NMR).
- As a chemical oxidizing agent for activated carbon production, as used in the Wentworth process. As the electrolyte in phosphoric-acid fuel cells.
- As an electrolyte in copper electropolishing for burr removal and circuit-board planarization.
- As a flux by metal workers and hobbyists (such as model railroaders) as an aid to soldering.
- In compound semiconductor processing, phosphoric acid is a common wet etching agent: for example, in combination with hydrogen peroxide and water it is used to etch InGaAs selective to InP.
- As a cleaner by construction trades to remove mineral deposits, cementitious smears, and hard-water stains.
- In hydroponics pH solutions to lower the pH of nutrient solutions. While other types of acids can be used, phosphorus is a nutrient used by plants, especially during flowering, making phosphoric acid particularly desirable.
- As a pH adjuster in cosmetics and skin-care products.
- As a dispersing agent in detergents and leather treatment.
- As an additive to stabilize acidic aqueous solutions within a wanted and specified pH range.

Storage and Side Effects of Phosphoric Acid
Store H3PO4 in containers made of corrosion-resistant materials. Sigma-Aldrich recommends storing phosphoric acid in a stainless-steel container with a resistant inner lining. Containers should always be stored upright to avoid leaks. Do not store in incompatible metals such as aluminium, the alloys of aluminium, and carbon steel. Store away from all incompatible chemicals and compounds. Keep away from food and foodstuffs. Ensure any solid material is kept dry. Do not reuse containers, even if they appear clean and empty.
Phosphoric Acid has been conducted so far are contradictory, but many have found links between consumption of cola-type soft drinks and kidney disease, kidney stones or osteoporosis. It is assumed that one of the causes of osteoporosis is the fact that this substance makes it difficult for the body to absorb calcium and magnesium, it binds to these minerals in the digestive tract, forming compounds that can not be absorbed. Osteoporosis and related fractures represent major public health problems. The lifetime risk of fracture exceeds 13 percent for men and 40 percent for women, and hip fractures have been linked with an excess mortality of up to 20%.