Introduction of Partition Chromatography
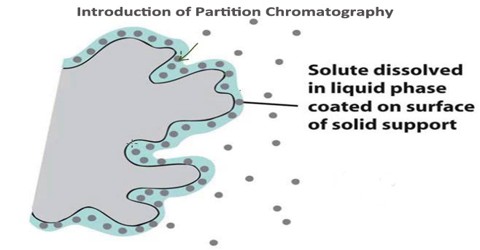
Partition chromatography is a type of chromatography. It is a method of separation in which the components present in the mixture get distributed more likely into two liquid phases because of differences in partition coefficients. It is based on differences in retention factor K as well as distribution coefficient Kd of the analytes using liquid for both stationary as well as the mobile phase. Partition chromatography can be divided into 1) liquid-liquid chromatography and 2) bonded-phase liquid chromatography.
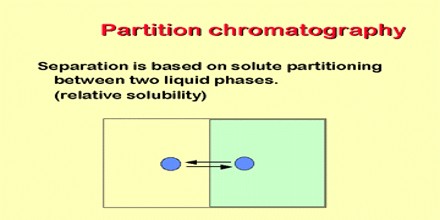
The partition chromatography is the basic principle involved in many separation techniques like high performance liquid chromatography and gas chromatography. Partition chromatography is basically understood as a method of separation of solutes utilizing the partition of the solutes between two liquid phases.
The process of separating mixtures of chemical compounds by passing them through a column that contained a solid stationary phase that was eluted with a mobile phase (column chromatography) was well known at that time. Chromatographic separation was considered to occur by an adsorption process whereby compounds adhered to a solid media and were washed off the column with a solvent, mixture of solvents, or solvent gradient. In contrast, Martin and Synge developed and described a chromatographic separation process whereby compounds where partitioned between two liquid phases similar to the separatory funnel liquid-liquid separation dynamic. This was an important departure, both in theory and in practice, from adsorption chromatography.
Chromatography
The term chromatography literally means color writing, and denotes a method by which the substance to be analyzed is poured into a vertical glass tube containing an adsorbent, the various components of the substance moving through the adsorbent at different rates, according to their degree of attraction to it, and producing bands of color at different levels of the adsorption column. The term has been extended to include other methods utilizing the same principle, although no colors are produced in the column.
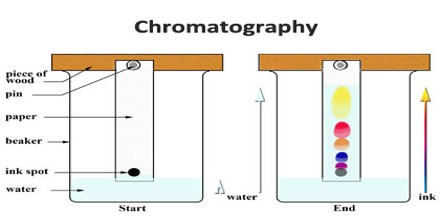
Classification of chromatographic techniques tends to be confusing because it may be based on the type of stationary phase, the nature of the adsorptive force, the nature of the mobile phase, or the method by which the mobile phase is introduced.
The technique is a valuable tool for the research biochemist and is readily adaptable to investigations conducted in the clinical laboratory. For example, chromatography is used to detect and identify in body fluids certain sugars and amino acids associated with inborn errors of metabolism.
Types and Applications of Partition Chromatography
Liquid-liquid chromatography uses liquid and the stationary phase is attached to a supporting matrix by physical means. As for example cellulose, starch or silica matrix is used to support water stationary phase and all these have the ability to bind physically as much as 50% water (w/v) and remain free floating powder. There are some advantages of liquid-liquid chromatography as for example silica, cellulose starch used are cheap, have a high capacity with broad selectivity.
However, there are some disadvantages too like elution of the sample may gradually remove the stationary phase thereby altering the chromatographic condition. To overcome these problems, bonded phases are used like in bonded phase liquid chromatography. Silica is derivatized to the immobilized stationary phase by using reaction with an organochlorosilane. Thus, with elution stationary phase will not change.
Normal phase liquid chromatography uses polar stationary phase and relatively non-polar mobile phase. Alkylamine bonded to silica is most commonly used as a stationary phase while organic solvents like hexane, heptanes, dichloromethane or ethyl acetate are used as mobile phase.
These solvents are used in such a way to form an eluotropic series based on their polarity with increasing polarity. The order of elution of analytes is performed in such a way that the least polar analyst is eluted first and the most polar analyst eluted at last.
Reverse phase liquid chromatography has many similarities with hydrophobic interaction chromatography. In this chromatography, the stationary phase is nonpolar and the mobile phase is relatively polar than the stationary phase. Both these, normal-phase liquid chromatography and reverse-phase liquid chromatography are the type of bonded phase liquid chromatography.