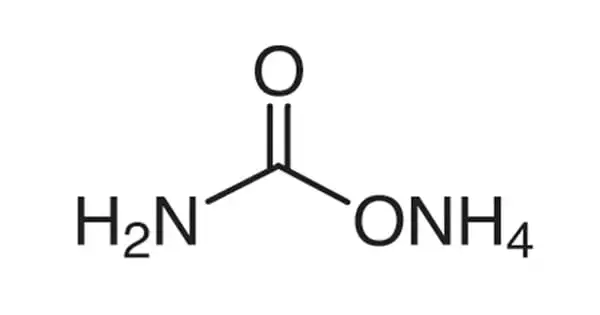
Ammonium carbamate is an organic compound with the formula [NH4][H2NCO2], which is composed of ammonium cation NH4+ and carbamate anion NH2COO. It is a white crystalline substance that is used as a fertilizer. It’s a white solid that’s quite soluble in water but not so much in alcohol.
At typical temperatures and pressures, ammonium carbamate can be created by the interaction of ammonia NH3 with carbon dioxide CO2, and it will slowly degrade to those gases. The most serious danger is the harm to the ecosystem. It serves as an intermediate in the commercial synthesis of urea (NH2)2CO, a vital fertilizer.
Properties
Ammonium carbamate is a salt NH2COONH4 present in commercial ammonium carbonate and generated as an intermediary in the production of urea by the interaction of ammonia and carbon dioxide. It is a colorless crystalline powder or white powder. The odor is similar to that of ammonia. The odor threshold is 5 ppm as NH3 (detection) and 46.8 ppm as NH3 (recognition).
- Density: 1.38 g/cm3 (20 °C)
- Melting point: 60°C (140 °F; 333 K) decomposes
- Boiling point: 58.76°C (estimate)
- Solubility in water: Freely soluble in water
- Solubility: Soluble in ethanol, methanol, liquid ammonia, formamide.
- Vapor pressure: 100 mm Hg ( 26.7 °C)

Solid-gas equilibrium
In a closed container solid ammonium carbamate is in equilibrium with carbon dioxide and ammonia.
[NH2CO2][NH4] ⇌ CO2 + 2NH3
Lower temperatures shift the equilibrium towards the carbamate.
At higher temperatures ammonium carbamate condenses into urea:
[NH2CO2][NH4] → (NH2)2CO + H2O
This reaction was first discovered in 1870 by Bassarov, by heating ammonium carbamate in sealed glass tubes at temperatures ranging from 130 to 140 °C.
Equilibrium in water
At ordinary temperatures and pressures, ammonium carbamate exists in aqueous solutions as an equilibrium with ammonia and carbon dioxide, and the anions bicarbonate, HCO−3, and carbonate, CO2−3. Indeed, solutions of ammonium carbonate or bicarbonate will contain some carbamate anions too.
Structure
The structure of solid ammonium carbamate has been confirmed by X-ray crystallography. The oxygen centers form hydrogen bonds to the ammonium cation.
Natural occurrence
Ammonium carbamate serves a key role in the formation of carbamoyl phosphate, which is necessary for both the urea cycle and the production of pyrimidines.
Preparation
From liquid ammonia and dry ice – Ammonium carbamate is prepared by the direct reaction between liquid ammonia and dry ice (solid carbon dioxide):
2NH3 + CO2 → [NH2CO2][NH4]
It is also prepared by reaction of the two gases at high temperature (450–500 K) and high pressure (150–250 bar).
From gaseous ammonia and carbon dioxide
Ammonium carbamate can alternatively be made by bubbling gaseous CO2 and NH3 in anhydrous ethanol, 1-propanol, or DMF at room temperature and pressure. The carbamate precipitates and is easily removed by filtration, and the liquid containing the unreacted ammonia can be reintroduced to the reactor. The lack of water precludes the creation of bicarbonate and carbonate, resulting in no ammonia loss.
Uses
Ammonium carbamate is a chemical that is used in the production of fertilizers. It functions as an ammoniac. It is vital in the creation of carbamoyl phosphate, which is required for the urea cycle and the production of pyrimidines. Furthermore, it is employed in the production of trimethylsilyl carbamate.
- Urea synthesis
Ammonium carbamate is a byproduct of the industrial urea synthesis process. A typical urea manufacturing plant may produce up to 1500 tons per day. in this reactor and can subsequently be dehydrated to urea using the equation:
[NH2CO2][NH4] → (NH2)2CO + H2O
- Pesticide formulations
The Environmental Protection Agency has also approved ammonium carbamate as an inert component in pesticide formulations containing aluminum phosphide. This herbicide is frequently used in agricultural storage areas to control insects and rodents.
- Laboratory
Although not as powerful as ammonia, ammonium carbamate can be employed as an ammoniating agent.