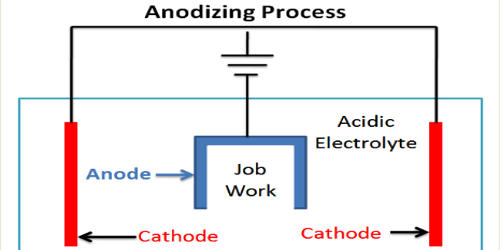
Anodizing is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts. It is an electrochemical process that converts the metal surface into a decorative, durable, corrosion-resistant, anodic oxide finish. The purpose of anodizing is to form a layer of aluminum oxide that will protect the aluminum beneath it.
The process is called anodizing because the part to be treated forms the anode electrode of an electrolytic cell. It is a process for finishing aluminum alloys that use electrolytic oxidation of the aluminum surface to produce a protective oxide coating. Anodising increases resistance to corrosion and wear, and provides better adhesion for paint primers and glues than bare metal does. Anodic films can also be used for several cosmetic effects, either with thick porous coatings that can absorb dyes or with thin transparent coatings that add interference effects to reflected light. In short, the main purposes of anodizing are corrosion resistance, abrasion/wear resistance, and cosmetics.
Anodizing is also used to prevent galling of threaded components and to make dielectric films for electrolytic capacitors. Aluminum is ideally suited to anodizing, although other nonferrous metals, such as magnesium and titanium, also can be anodized. Anodic films are most commonly applied to protect aluminum alloys, although processes also exist for titanium, zinc, magnesium, niobium, zirconium, hafnium, and tantalum. Anodization is a synthesis technique where a protective oxide layer of thickness 5–25 μm is developed on the surface of the metal by electrolytic oxidation of metal surface in the presence of an acid. Iron or carbon steel metal exfoliates when oxidized under neutral or alkaline micro-electrolytic conditions; i.e., the iron oxide forms by anoxic anodic pits and large cathodic surface, these pits concentrate anions such as sulfate and chloride accelerating the underlying metal to corrosion.
Benefits include:
- An anodized finish will not break down or decompose,
- The finish is non-toxic,
- It is heat-resistant to 1,221°F (the melting point of aluminum),
- Anodizing uses simple water-based chemicals that can be treated easily and that release no harmful by-products,
- The liquid by-products can be recycled and returned to the process.
Anodizing is accomplished by immersing the aluminum into an acid electrolyte bath and passing an electric current through the medium. Carbon flakes or nodules in iron or steel with high carbon content (high-carbon steel, cast iron) may cause an electrolytic potential and interfere with coating or plating. Ferrous metals are commonly anodized electrolytically in nitric acid or by treatment with red fuming nitric acid to form hard black Iron(II, III) oxide. This oxide remains conformal even when plated on wiring and the wiring is bent. Anodizing is, therefore, a matter of highly controlled oxidation the enhancement of a naturally occurring phenomenon.