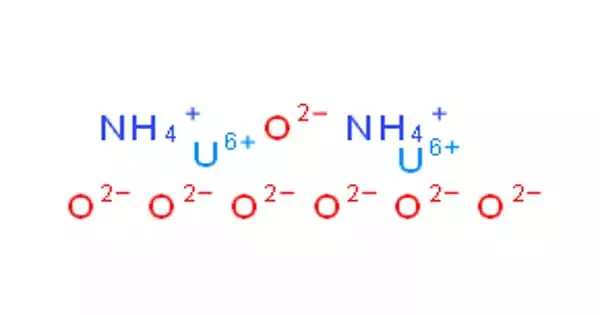
Ammonium diuranate (ADU) is a uranium chemical compound. Uranium is a chemical element with the atomic number 92 and the symbol U. It is a natural component of rocks, soil, air, and water, and it can be found in nature in the form of minerals. It is one of the intermediary chemical forms of uranium created during yellowcake manufacture, with the chemical formula [(NH4)2U2O7]. The term “yellowcake,” which was initially applied to this vivid yellow salt, today refers to combinations of uranium oxides that are rarely yellow.
It also is an intermediate in mixed-oxide (MOX) fuel fabrication. Although it is usually called “ammonium diuranate” as though it has a “diuranate” ion, this is not necessarily the case. It can also be called diammonium diuranium heptaoxide. The structure is said to be similar to that of uranium dioxide dihydrate.
Ammonium diuranate (ADU) and its heating product uranium oxides (UO3 + U3O8) are critical intermediates in the manufacturing of nuclear fuel. In both laboratory and industrial sizes, it has been precipitated from nitric acid solutions by the addition of anhydrous ammonia. This method yielded more dense and swiftly filtered precipitates than the addition of aqueous ammonia or slurried calcium hydroxide. The filtrates obtained using the anhydrous ammonia procedure had less uranium than those obtained after the addition of the other reagents.
It is precipitated by adding aqueous ammonium hydroxide after uranium extraction with tertiary amines in kerosene. This precipitate is then thickened and centrifuged before being calcined to uranium oxide. Canadian practice favors producing uranium oxide from ammonium diuranate rather than uranyl nitrate, as is the case elsewhere.
Application
Ammonium diuranate was once used to make colored glazes in ceramics. However, because uranate decomposes to uranium oxide when burnt, it was only employed as a lesser cost material than completely purified uranium oxide.
In general, magnesio-thermic reduction of UF4 produces uranium metal, which is used to make fuel for research reactors in India. The performance of the magnesio-thermic reaction and recovery, as well as the purity of the uranium, are heavily dependent on the characteristics of UF4. Because ammonium diuranate (ADU) is the first product in the process flow-sheet in powder form, the qualities of UF4 are dependent on the properties of ADU. ADU is typically manufactured using uranyl nitrate solution (UNS) for natural uranium metal production and uranyl fluoride solution (UFS) for low enriched uranium metal synthesis.