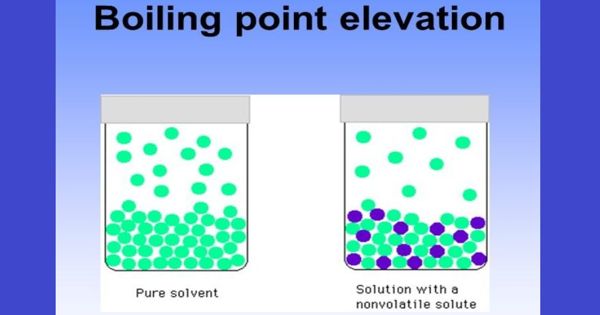
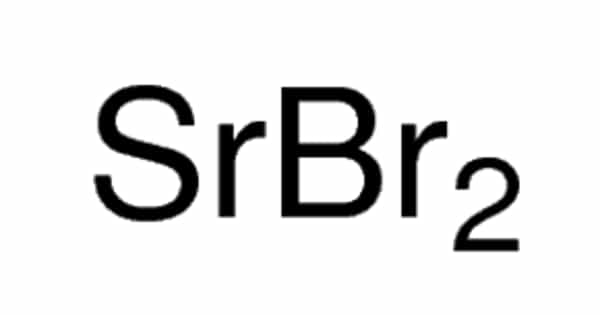The boiling point of a solvent will increase when a solute is dissolved in it. This is referred to as boiling point elevation. Boiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. It refers to the increase in the boiling point of a solvent upon the addition of a solute. The boiling point of the solvent above a solution will be greater than the boiling point of the pure solvent whether the solution contains a non-volatile solute or a volatile solute.
The boiling point elevation is the difference in temperature between the boiling point of the pure solvent and that of the solution.
The elevation of the boiling point is directly dependent on the amount of solute present in the solution, but it is not based on the identity of the solute, so it is considered a colligative property. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. The boiling point can be measured accurately using an ebullioscope. When a non-volatile solute is added to a solvent, the resulting solution has a higher boiling point than that of the pure solvent. For example, the boiling point of a solution of sodium chloride (salt) and water is greater than that of pure water.
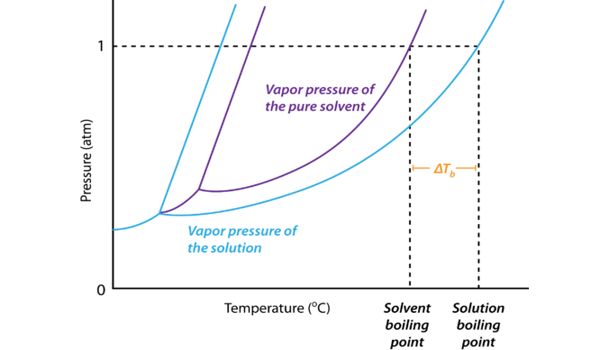
Why Does Boiling Point Elevation Occur –
The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of its surrounding environment. The boiling point elevation is a colligative property, which means that it is dependent on the presence of dissolved particles and their number, but not their identity. A solvent’s vapor pressure will lower when a solute is added. This happens because of the displacement of solvent molecules by the solute. It is an effect of the dilution of the solvent in the presence of a solute. It is a phenomenon that happens for all solutes in all solutions, even in ideal solutions, and does not depend on any specific solute-solvent interactions. This means that some of the solvent molecules at the surface of the liquid are replaced by the solute; it can occur in both electrolytic and non-electrolytic solutions.
The boiling point elevation happens both when the solute is an electrolyte, such as various salts, and a nonelectrolyte. The lower number of solvent molecules at the surface means that fewer will evaporate, and thus the vapor pressure is lowered. In thermodynamic terms, the origin of the boiling point elevation is entropic and can be explained in terms of the vapor pressure or chemical potential of the solvent. For the vapor pressure to equal the atmospheric pressure, a higher temperature is required, and a higher boiling point is observed. In both cases, the explanation depends on the fact that many solutes are only present in the liquid phase and do not enter into the gas phase (except at extremely high temperatures).
Information Source: