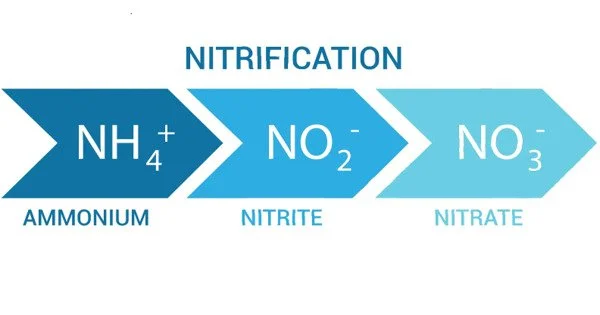
Nitrification is the biological oxidation of ammonia to nitrate via the intermediary nitrite. Nitrification is an important step in the nitrogen cycle in soil. It is a biological process in the nitrogen cycle that involves the conversion of ammonia (NH₃) or ammonium ions (NH₄⁺) into nitrite (NO₂⁻) and then further into nitrate (NO₃⁻). This process is primarily carried out by certain species of bacteria known as nitrifying bacteria.
Complete nitrification can occur through distinct species or wholly within one organism, as with comammox bacteria. The conversion of ammonia to nitrite is typically the rate-limiting step in nitrification. Nitrification is an aerobic process carried out by tiny groups of autotrophic bacteria and archaea.
The nitrification process occurs in two sequential steps:
(1) Ammonia oxidation to nitrite:
Ammonia (NH₃) is oxidized to nitrite (NO₂⁻) by ammonia-oxidizing bacteria (AOB). The key bacterium involved in this step is typically Nitrosomonas.
NH 3 +O 2 →NO 2−+H 2O+2e −
(2) Nitrite oxidation to nitrate:
Nitrite (NO₂⁻) is further oxidized to nitrate (NO₃⁻) by nitrite-oxidizing bacteria (NOB). Nitrobacter is an example of a bacterium that performs this conversion.
NO 2−+O2 →NO3− +H 2 O+2e −
Nitrification is an important process in soil and aquatic ecosystems because it plays an important part in the nitrogen cycle. It converts ammonia, which is frequently a byproduct of organic matter decomposition, animal waste, or fertilizer application, into forms that plants may readily absorb. Nitrate, in instance, is a type of nitrogen that plants may absorb and use for growth. However, excessive nitrification, which results in high nitrate levels in water bodies, can contribute to water pollution and pose environmental concerns.