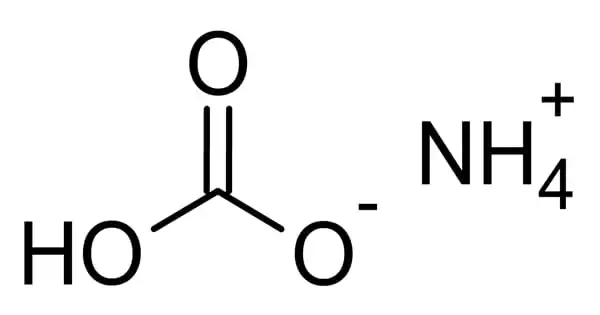
The inorganic compound ammonium bicarbonate has the formula (NH4)HCO3. Ammonium hydrogen carbonate is also known as Monoammonium carbonate or Ammonium hydrogen carbonate. It has a strong ammonia odor and looks as a crystalline solid white. It dissolves well in water but is insoluble in most organic solvents.
It is the bicarbonate salt of the ammonium ion in chemistry. It is the bicarbonate salt (HCO3) of the ammonium ion (NH4+) that easily degrades to CO2 (carbon dioxide), ammonia, and water. It’s a colorless substance that easily degrades into carbon dioxide, water, and ammonia.
Properties
Ammonium hydrogen carbonate is a white crystalline substance with an ammonia-like odor. It is water-soluble but not soluble in ethanol, acetone, alcohol, or benzene. It is harmful to the environment, and quick action should be done to halt its spread. It is frequently utilized in the food processing industry.
- Molecular weight: 79.056 g/mol
- Density: 1.586 g/dm3
- Flash point: Non-flammable
- Melting Point: 41.9 °C

Production
Ammonium bicarbonate is produced by combining carbon dioxide and ammonia:
CO2 + NH3 + H2O → (NH4)HCO3
Because ammonium bicarbonate is thermally unstable, the reaction solution is kept cold, allowing the result to precipitate as a white solid. In 1997, about 100,000 tons were manufactured in this manner.
Ammonia gas passed through a strong aqueous solution of the sesquicarbonate (a 2:1:1 mixture of (NH4)HCO3, (NH4)2CO3, and H2O) transforms it to normal ammonium carbonate ((NH4)CO3), which can be obtained in crystalline form from a solution made at around 30 °C. When exposed to air, this chemical emits ammonia and reverts to ammonium bicarbonate.
Reactions
It dissolves in water to give a mildly alkaline solution. It is insoluble in acetone and alcohols.
Ammonium bicarbonate decomposes above about 36 °C into ammonia, carbon dioxide, and water in an endothermic process and so causes a drop in the temperature of the water:
NH4HCO3 → NH3 + H2O + CO2.
When treated with acids, ammonium salts are also produced:
NH4HCO3 + HCl → NH4Cl + CO2 + H2O.
Reaction with base produces ammonia.
It reacts with sulfates of alkaline-earth metals precipitating their carbonates:
CaSO4 + 2 NH4HCO3 → CaCO3 + (NH4)2SO4 + CO2 + H2O.
It also reacts with alkali metal halides, giving alkali metal bicarbonate and ammonium halide:
NH4HCO3 + NaCl → NH4Cl + NaHCO3;
NH4HCO3 + KI → NH4I + KHCO3;
NH4HCO3 + NaBr → NH4Br + NaHCO3.
Natural occurrence
The chemical occurs naturally as the extremely uncommon mineral teschemacherite. It is found in minute amounts in nitrogenous organic materials, along with a variety of other ammonium salts.
Uses
In the food business, ammonium bicarbonate is used as a leavening ingredient for flat baked goods such as cookies and crackers. Prior to the availability of modern-day baking powder, it was widely utilized in the home.
- It is used in the food industry as a food additive.
- It is used in fire extinguishers.
- It is used in the manufacturing of dyes.
- It is used as a fertilizer.
- It is used to produce ammonium salt.
- It is used in the making of paints.
- It is used in the manufacturing of ceramics.
Safety
Ammonium bicarbonate is a skin, ocular, and respiratory system irritant. Short-term health effects from ammonium bicarbonate exposure may develop immediately or shortly after exposure. Ammonium bicarbonate can irritate the nose, throat, and lungs, resulting in coughing, wheezing, and/or shortness of breath. Repeated exposure may result in bronchitis, accompanied by a cough and/or shortness of breath. Health consequences can start months or years after ammonium bicarbonate exposure and can endure for months or years.