The objects around us are made of matter, which comes in three varieties: element, compound, and mixture. Chemistry is the branch of science that studies these three types. Elements are substances that cannot be broken down into simpler substances.
A compound is a chemical combination of elements that have been bonded together in a specific proportion. The physical combination of substances bonded together in any proportion is referred to as a mixture. The compound results in the formation of a new substance, whereas the mixture does not result in the formation of a new substance.
The compound is a pure substance, whereas the mixture is impure. Mixtures can be homogeneous or heterogeneous, whereas compounds are always homogeneous. Many science students struggle to understand the distinction between compound and mixture, so we’ve simplified it for you here.
Difference between Compound and Mixture –

COMPOUND
- A compound is a substance formed by the chemical combination of two or more elements. The term compound refers to a substance formed by chemically combining two or more substances in a specific weight ratio.
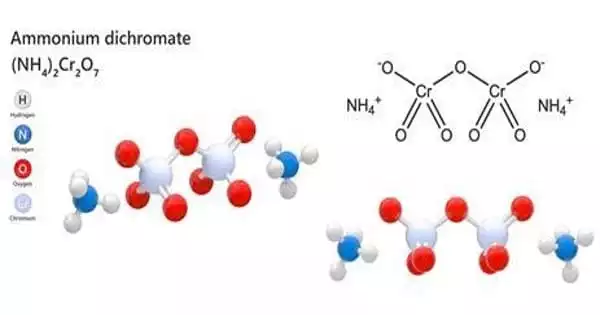
- A compound is a substance that is formed as a chemical blend of various elements in a specific proportion by weight. It is an entirely new substance with properties distinct from those of its constituent substances. Water, salt, carbon dioxide, sodium chloride, and so on.
- The ingredients in a compound are present in a specific proportion. The properties of a compound are the same as the properties of its constituents. The compound is a pure substance that only contains one type of molecule.
- A compound is the combination of several elements so that the atoms in the elements are held together by a chemical bond that cannot be easily broken. Bonds are formed when electrons are shared among atoms.
- The constituents of a compound can be separated only by chemical or electro-chemical reactions. Compounds are boiled or melted at a definite temperature.
MIXTURE
- Mixture refers to the physical blending of two or more substances into one. A mixture is defined as a substance formed by physically combining two or more substances into one.
- A mixture is formed when two or more substances are combined in any ratio such that no chemical reaction occurs. For instance, sand and water, sugar and salt, air, and so on.
- The constituents in a mixture are present in varying proportions. In contrast to a mixture, where the properties of the ingredients and the mixture are the same. A mixture is an impure substance made up of different types of molecules.
- The properties of the components of a mixture are retained even after they have been mixed as a solution, suspension, or colloids. Physical means should be able to separate the combination back to normal.
- The components of the mixture can be bifurcated by physical methods. Mixtures do not have a fixed melting and boiling point.