To make plastic, small monomer molecules are strung together like beads in a necklace to form long polymer chains. However, not all plastics—or the polymers that make them up—are created equal. The more durable the material, the longer and stronger the polymer.
Cornell researchers took a mediocre monomer and used a special catalyst to create a tougher polymer capable of forming long chains. With an acid catalyst, the polymer can be easily depolymerized back to the monomer state, resulting in a chemically recyclable thermoplastic that competes with the most popular plastics, polyethylene, and polypropylene.
The team’s paper, “Chemically Recyclable Thermoplastics from Reversible-Deactivation Polymerization of Cyclic Acetals,” was published in Science. The co-lead authors are former postdoctoral researchers Brooks Abel and Rachel Snyder, Ph.D. ’21.
Polymer allows for more durable recyclable thermoplastics. To synthesize plastic, small monomer molecules need to be strung together like beads in a necklace, creating long polymer chains.
“Ideally, the perfect polymer is one that has really high initial stresses and then undergoes really good elongation,” said Geoffrey Coates, the paper’s senior author and a Tisch University Professor in the College of Arts and Sciences. “Polyethylene and polypropylene, which you’ve probably heard of, have excellent properties. Many new polymers do not compare favorably to these tried-and-true ones. Our polymer ranks in the middle of the field. It’s been around for 60 or 70 years, but no one has been able to make really long chains of it with good properties.”
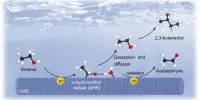
In an unexpected twist, the discovery came from the Coates Group’s involvement with the Joint Center for Energy Storage Research, an interdisciplinary collaboration launched by the United States Department of Energy to realize next-generation batteries. Coates and his colleagues were working to create sustainable polymers that could be used in energy storage and conversion materials when they realized their polymer, poly(1,3-dioxolane) or PDXL, was well-suited for creating a thermoplastic—a material that can be melted, recycled, and remolded.
The polymer was created by the researchers using dioxolane, a cyclic acetal monomer derived from potentially bio renewable formaldehyde and ethylene glycol feedstocks. Polyacetals are excellent candidates for the production of recyclable thermoplastics because they are stable at temperatures above 300 degrees Celsius but depolymerize at relatively low temperatures—typically below 150 degrees Celsius—in the presence of an acid catalyst. They are also inexpensive and can be obtained biologically. Polyacetals, on the other hand, have not previously been used because the polymer chains are generally too short to achieve the mechanical strength required for commercial applications.

“We wanted to develop a new method of producing polyacetals that would allow us to control the length of the polymer chains,” Abel explained. “In the end, we were able to create really high molecular weight polyacetals that were surprisingly ductile and strong in comparison to their more brittle, low molecular weight counterparts.”
“You need a really high molecular weight if you want to make a cup that doesn’t crack when you flex it,” Coates said. The researchers were able to connect the monomers into long chains of PDXL with high molecular weight and tensile strength using a process known as reversible-deactivation cationic ring-opening polymerization.
The resulting thermoplastic is strong and flexible enough to be used in large-scale applications like the packaging. The team demonstrated this capability by developing a number of prototype items, including protective pouches, molded packaging, and inflatable air pillows similar to those used by Amazon to pad their boxes.
“Right now, nearly 40% of all plastic is produced for packaging products that are used briefly and then discarded,” Snyder explained. “PDXL has the necessary strength for packaging, but rather than discarding it, we can collect and repurpose it through a highly efficient chemical recycling process. As a result, it is an ideal candidate for a circular polymer economy.”
The recycling process is so effective that PDXL can even be depolymerized from complex plastic waste mixtures. The researchers combined PDXL with other common plastics such as polyethylene terephthalate, polyethylene, and polystyrene. They were able to recover 96 percent of the pure dioxolane monomer after using a reusable acid catalyst and heat, demonstrating that it can be easily isolated from common contaminants such as dyes and plasticizers. The recovered monomer was then repolymerized to PDXL, demonstrating the cyclical nature of polyacetal chemical recycling.
This highlights the polymer’s most important feature: its long-term viability. “These plastics require a significant amount of fossil fuels to manufacture, and the carbon footprint of common polyethylene or polypropylene is extremely high. So we need to improve how we make them “Coates stated. “If you have a way to chemically recycle the polymer, it won’t end up in the ocean, right? And then, instead of expending all this energy to extract oil from the ground and break it up into small pieces, all we have to do is heat up the polymer, and presto, we have a monomer again.”