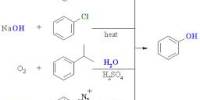
One of the most useful industrial sodium compounds is sodium hydroxide (NaOH), also known as lye and caustic soda. It is one of the household market’s most strong base alkalis (high pH value). Sodium hydroxide is an exceptionally acidic base and antacid that breaks down proteins at normal encompassing temperatures and may cause extreme synthetic consumes. It is profoundly dissolvable in water and promptly retains dampness and carbon dioxide from the air. Its solubility in water is 111% by weight and vapor pressure of 0mmHg (NIOSH, 1994). As an aqueous solution referred to as caustic soda, soda lye, or simple as lye, sodium hydroxide is widely available. It has numerous applications, including acid neutralization; the processing of paper, textiles, plastics, corrosives, dyestuffs, paint, paint remover, and soap; petroleum refining; electroplating; cleaning of metals; laundering; and washing of dishes.
At room temperature, sodium hydroxide is a white crystalline solid; it is odorless and absorbs humidity from the air. When mixed in water, it releases heat that is necessary for combustible compounds to ignite. It is used as a drain and oven cleaner, and in soap making, it saponifies fats. It must be used cautiously because it is also capable of generating extreme skin burns. Sodium hydroxide is commonly used alongside neutral water and acidic hydrochloric acid to illustrate the pH scale to chemistry students as one of the simplest hydroxides. Below are some important uses of sodium hydroxides in various fields:

Some important uses of Sodium Hydroxide
Sodium hydroxide is used for the manufacture of soaps and a number of household and industrial detergents. By combining chlorine and sodium hydroxide, chlorine bleach is generated. Drain cleaners that contain sodium hydroxide turn soap that dissolves in water into fats and grease that can clog pipes.
In the pharmaceutical industry, sodium hydroxide is used to produce various medications such as aspirin, which is a pain reliever, medicines that reduce cholesterol, and medicines that prevent blood clots. It is used in the paper industry for the processing of paper. In the aluminum industry, it is used in the processing of aluminum products. It is also used in the industry for detergents.
Sodium hydroxide is used in the energy sector in the manufacture of fuel cells. For a variety of applications, like transportation; material handling; and stationary, portable, and emergency backup power applications, fuel cells act like batteries to generate electricity cleanly and efficiently. Manufactured with sodium hydroxide, epoxy resins are used in wind turbines.
Sodium hydroxide is used in the petroleum industry as an additive in drilling mud to increase alkalinity in bentonite mud systems, increase mud viscosity, and neutralize any acid gas (such as hydrogen sulfide and carbon dioxide) that may be found as drilling progresses during geological formation.
The colorless liquid is sodium hydroxide; it’s much denser than water. It can cause discomfort in the eyes, mucous membranes, and skin when it comes into contact. It’s corrosive to metals. They are glazed with a sodium hydroxide solution until they are baked to produce crispy German lye rolls. Another usage is in Salt spray testing where pH needs to be controlled. For pH balance, sodium hydroxide is used with hydrochloric acid. The corrosive agent used in the regular neutral pH salt spray test is the resulting salt, NaCl.
Sodium hydroxide is used less commonly as a toothpaste component. Other uses include the removal of chemicals from vegetables and fruits, caramel color manufacturing, processing of cocoa and chocolate, ice cream thickening, and soft drink processing. Olives can be soaked in sodium hydroxide to soften them. Sodium hydroxide is also used in wood bleaching and cleaning to process raw materials for wood products, such as cabinets and furniture.
To extract alumina from naturally occurring rocks, sodium hydroxide is used. Alumina is used to produce aluminium, including foil, cans, kitchen utensils, beer kegs and airplane parts, and a number of items. In building and construction, aluminum is used in materials that allow facades and window frames to be constructed.
Sodium hydroxide is used to digest tissues in a similar manner, as in a procedure that was used at one time for farm animals. In this process, a carcass was put in a sealed chamber and a mixture of sodium hydroxide and water was applied (which breaks the chemical bonds that keep the flesh intact). This gradually transforms the body into a coffee-like liquid, and bone hulls, which could be crushed between one’s fingers, are the only solid that remains.
Other applications include the recovery of rubber, the dissolution of casein in the manufacture of plastics, the refining of vegetable oils, the processing of textiles as an eluent in ion chromatography, etching, and electroplating, and as a laboratory reagent. In many organic synthesis and base-catalyzed reactions, sodium hydroxide is also used as a strong base. It is used for the manufacturing of rayon, spandex, explosives, epoxy resins, paints, ceramics, and glass. Zinc and lead, which dissolve to give sodium zincate and sodium plumbate in condensed sodium hydroxide solutions, are other amphoteric metals.
Information Sources: