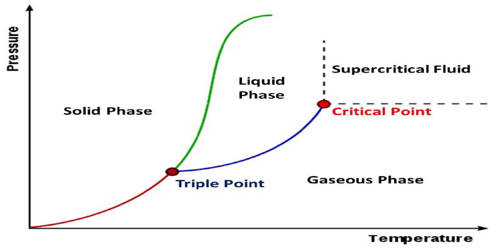
Neon is a chemical element with the symbol Ne and atomic number 10. It is an inert gas of Group 18 of the periodic table, used in electric signs and fluorescent lamps. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It occurs naturally in the atmosphere, but only in very small amounts. It changes from a gas to a liquid at -245.92°C (-410.66°F) and from a liquid to a solid at -248.6°C (-415.5°F). Its density is 0.89994 grams per liter. It is obtained by liquefaction of air and separated from the other gases by fractional distillation.
It was discovered (along with krypton and xenon) in 1898 as one of the three residual rare inert elements remaining in dry air, after nitrogen, oxygen, argon, and carbon dioxide were removed. The abundance of neon in normal air is 18.2 parts per million (0.0182 percent). Neon was the second of these three rare gases to be discovered and was immediately recognized as a new element from its bright red emission spectrum. It is usually found in the form of a gas with molecules consisting of a single Neon atom. Neon is a rare gas that is found in the Earth’s atmosphere at 1 part in 65,000. The compounds of neon currently known include ionic molecules, molecules held together by van der Waals forces, and clathrates.
- atomic number: 10
- atomic weight: 20.183
- melting point: −248.67 °C (−415.5 °F)
- boiling point: −246.048 °C (−411 °F)
- density: (1 atm, 0° C) 0.89990 g/litre
- oxidation state: 0
Neon was discovered (1898) by the British chemist’s Sir William Ramsay and Morris W. Travers as a component of the most volatile fraction of liquefied crude argon obtained from air. During cosmic nucleogenesis of the elements, large amounts of neon are built up from the alpha-capture fusion process in stars. Although neon is a very common element in the universe and solar system, it is rare on Earth. It composes about 18.2 ppm of air by volume and a smaller fraction in Earth’s crust. It has the smallest temperature range (2.6 degrees C or 4.7 degrees F) for which it is a liquid, according to Chemicool.
Uses
- The largest use of neon is in making the ubiquitous ‘neon signs’ for advertising. It gives a distinct reddish-orange glow when used in low-voltage neon glow lamps, high-voltage discharge tubes, and neon advertising signs.
- It is used in some plasma tube and refrigerant applications but has few other commercial uses. It is commercially extracted by the fractional distillation of liquid air.
- It is also used to make high-voltage indicators and switching gear, lightning arresters, diving equipment and lasers.