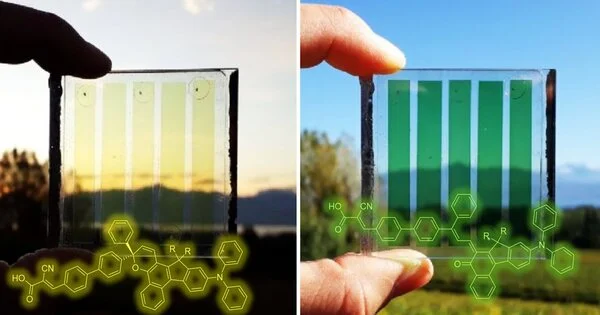
A dye-sensitized solar cell (DSSC or Grätzel cell) is a low-cost solar cell that belongs to the thin-film solar cell family. A photoelectrochemical system is formed by a semiconductor formed between a photo-sensitized anode and an electrolyte. These are thin-film photovoltaic devices that transform light into electricity. Because of their low cost of fabrication, ease of manufacturing, and potential for flexible and transparent applications, they are a promising alternative to traditional silicon-based solar cells.
The modern version of a dye solar cell, also known as the Grätzel cell, was originally co-invented in 1988 by Brian O’Regan and Michael Grätzel at UC Berkeley and this work was later developed by the aforementioned scientists at the École Polytechnique Fédérale de Lausanne (EPFL) until the publication of the first high efficiency DSSC in 1991. Michael Grätzel has been awarded the 2010 Millennium Technology Prize for this invention.
The DSSC has a number of appealing characteristics: it is simple to manufacture using traditional roll-printing techniques, it is semi-flexible and semi-transparent, allowing for a variety of uses not possible with glass-based systems, and the majority of the materials used are low-cost. In practice, it has proven difficult to eliminate a number of expensive materials, most notably platinum and ruthenium, and the liquid electrolyte presents a significant challenge to developing a cell that can be used in all weather conditions.
The concept of DSSCs was first introduced by Michael Grätzel and Brian O’Regan at the École Polytechnique Fédérale de Lausanne in the early 1990s. The basic structure of a DSSC consists of several key components:
- Transparent Conductive Substrate: Typically made of glass or plastic coated with a transparent and conductive material such as fluorine-doped tin oxide (FTO) or indium tin oxide (ITO). This substrate allows light to enter the active layers of the cell.
- Working Electrode (Photoanode): A layer of titanium dioxide (TiO2) nanoparticles is applied to the transparent conductive substrate. The TiO2 layer is coated with a photosensitive dye that absorbs light and initiates the electron transfer process.
- Photosensitive Dye: The dye is the heart of the DSSC, capturing light energy and converting it into electrical charges. Organic dyes and inorganic metal-organic complexes are the most common types of dyes.
- Electrolyte: An electrolyte solution containing redox couples is used to shuttle electrons between the working electrode and the counter electrode. The redox couple typically consists of an iodide/triiodide (I−/I3−) or cobalt-based electrolyte.
- Counter Electrode (Cathode): The counter electrode is typically a platinum-coated conductive glass, which acts as a catalyst for the reduction of the redox couple from the electrolyte.
The operation of a DSSC involves several steps:
- Light Absorption: When sunlight (photons) strikes the DSSC, the photosensitive dye absorbs the light and becomes excited. The dye molecules enter a higher energy state, releasing electrons in the process.
- Electron Injection: The excited electrons from the dye are injected into the conduction band of the TiO2 nanoparticles in the working electrode.
- Electron Transport: The injected electrons move through the TiO2 nanoparticles, while the dye returns to its ground state.
- Electrolyte Interaction: The electrons are transferred from the TiO2 to the electrolyte, which regenerates the dye by accepting the electrons.
- Electron Collection: The electrons travel through an external circuit from the working electrode to the counter electrode, generating an electric current.