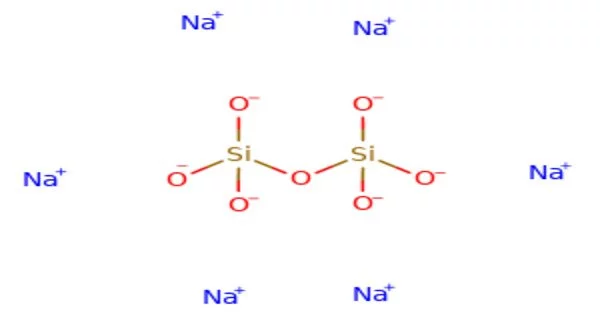
Sodium pyrosilicate is the chemical formula Na6Si2O7. It is a sodium silicate, specifically a pyrosilicate, which is a salt of the unstable pyrosilicic acid H6Si2O7. It is used in laundry detergents, dishwasher detergents, and other cleaning agents. Its strong alkaline nature and ability to emulsify oils and fats help to remove dirt, grease, and stains from fabrics and surfaces.
Properties
- Appearance: It is typically a white or light-colored solid.
- Solubility: It is sparingly soluble in water, meaning it dissolves to a limited extent.
- Heat resistance: Like other silicates, it exhibits thermal stability and can withstand high temperatures without decomposition.
Structure
The anhydrous solid has the triclinic crystal structure, with space group P1 (a = 5.8007(8) Å, b = 11.5811(15) Å, c = 23.157(3) Å, α = 89.709(10)°, β = 88.915(11)°, γ = 89.004(11)°, V = 1555.1(4) Å3, Z = 8, Dx = 2.615 g·cm−3, μ(Mo‐Kα) = 7.94 cm−1). The Si2O6+7 anions are arranged in layers parallel to the (100) plane, with the sodium cations distributed in 24 distinct crystallographic positions, coordinated by 4 to 6 near oxygen atoms. Some of the 4-coordinated sodium atoms can be interpreted as parallel columns of edge-sharing NaO 4 tetrahedra. The columnar arrangement forms tunnels that house the remaining sodium cations. Twinning at a microscopic scale simulates a much larger monoclinic C centered lattice (V′ = 6220 Å3, Z = 32).
Application
Because of its thermal and chemical properties, sodium pyrosilicate may find use in a variety of industrial applications, including the production of ceramics, glazes, and coatings. Sodium pyrosilicate is used as a cleaning and anti-corrosion agent for metal surfaces in the automotive and metalworking industries. It aids in the removal of oils, greases, and other contaminants, resulting in improved coating adhesion and protection against rust and corrosion.
As a flocculating and coagulating agent, sodium pyrosilicate is used in water treatment processes. It helps remove suspended particles and impurities from water, making it suitable for a wide range of industrial and municipal applications.
Sodium pyrosilicate is used as a binder and adhesive in the construction industry to make ceramic tiles and building materials. It helps improve the adhesion of tiles and reduces water absorption.