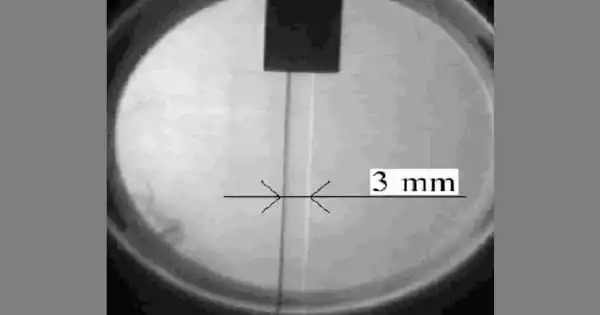
The solid-state of the element hydrogen is created by lowering the temperature below the melting point of hydrogen, which is 14.01 K (−259.14 °C; −434.45 °F). With a density of 0.086 g/cm3, solid hydrogen is one of the lowest-density solids.
Hydrogen is one of the cleanest energy, with the ability to emit no carbon dioxide. Hydrogen storage is a difficult stage in hydrogen energy use. The safety, affordability, and transportation of compressed and liquified hydrogen all pose challenges to the widespread use of hydrogen energy. Because of its great storage and transit capabilities, the chemical absorption of hydrogen in solid hydrogen storage materials is a viable hydrogen storage technology.
Molecular solid hydrogen
At low temperatures and pressures up to 400 GPa, hydrogen condenses into a sequence of solid phases composed of distinct H2 molecules. At low temperatures and pressures, phase I is composed of a hexagonal close-packed array of freely rotating H2 molecules. When pressure is increased at low temperature, a transition to Phase II occurs at pressures of up to 110 GPa. Phase II is a broken-symmetry configuration in which the H2 molecules cannot freely rotate. When the pressure is increased further at low temperature, a Phase III is reached at around 160 GPa. When the temperature rises to a few hundred degrees Celsius and the pressure rises above 220 GPa, a transition to Phase IV occurs.
Identifying the atomic structures of the various phases of molecular solid hydrogen is extremely difficult because hydrogen atoms interact with X-rays very weakly and only small samples of solid hydrogen can be achieved in diamond anvil cells, so X-ray diffraction provides very limited information about the structures. Nonetheless, phase transitions can be discovered by checking for rapid changes in the Raman spectra of samples.
A combination of experimental Raman spectra and first-principles modeling can also be used to infer atomic structures. Density functional theory simulations were utilized to identify potential atomic configurations for each phase. These candidates have low free energy and Raman spectra that match the observed spectra. Quantum Monte Carlo methods were then used, along with a first-principles treatment of anharmonic vibrational effects, to obtain the relative Gibbs free energies of these structures, and thus a theoretical pressure-temperature phase diagram that is in reasonable quantitative agreement with experiment.
On this basis, Phase II is thought to be a P21/c symmetry molecular structure; Phase III is (or is similar to) a C2/c symmetry structure consisting of flat layers of molecules in a distorted hexagonal arrangement; and Phase IV is (or is similar to) a Pc symmetry structure consisting of alternate layers of strongly bonded molecules and weakly bonded graphene-like sheets.