The inorganic compound with the formula PbO2 is lead dioxide, also known as Lead(IV) oxide. In the activity of the lead-acid storage battery, it is a brown material necessary. It is an oxide where lead is in a state of +4 oxidation. Lead dioxide tends to be brown, hexagonal, and insoluble in water crystals. In two crystalline forms, it exists. In electrochemistry, it has many significant applications, particularly as the positive plate of lead-acid batteries.
Lead dioxide is a crystalline solid or powder that is dark brown. It has two main polymorphs, alpha, and beta, naturally occurring as scrutinyite and plattnerite rare minerals, respectively. Although the beta type was described in 1845, alpha-PbO2 was first recognized in 1946 and discovered in 1988 as a naturally occurring mineral. Practically insoluble in water; also insoluble in alkalis; reasonably soluble in hydrochloric acid and also in a mixture of nitric acid-hydrogen peroxide; dissolved slowly in acetic acid.
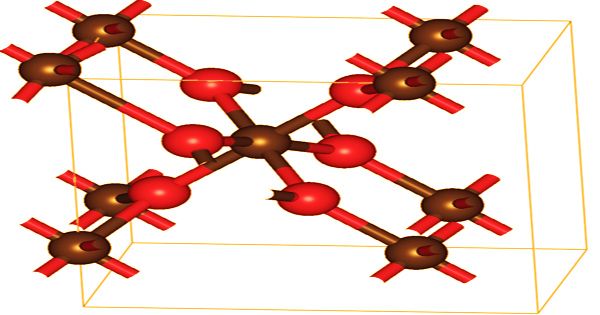
Crystal structure of Lead Dioxide
As follows, lead dioxide decomposes in the air when heated:
24 PbO2 → 2 Pb12O19 + 5 O2
Pb12O19 → Pb12O17 + O2
2 Pb12O17 → 8 Pb3O4 + O2
2 Pb3O4 → 6 PbO + O2
In nature, lead dioxide occurs as the mineral plattnerite. In manufacturing dyes and intermediates, it is used as an oxidizing agent. The stoichiometry of the end product can be regulated by temperature changes; for instance, the first stage occurs at 290 °C, the second at 350 °C, the third at 375 °C, and the fourth at 600 °C in the above reaction. Furthermore, Pb2O3 can be obtained under an oxygen pressure of 1,400 atm (140 MPa) by decomposing PbO2 at 580–620 °C.
Thermal decomposition of lead dioxide, therefore, is a common way of creating different lead oxides. It is also used in tournaments, pyrotechnics, and explosives as a source of oxygen. In matches, the oxide is mixed as an ignition surface with amorphous phosphorus. It is also used for the manufacture of lead pigments, polymers of liquid polysulfide, and rubber substitutes. Lead dioxide is a mainly acidic amphoteric compound. In solid bases, it dissolves to form the hydroxyplumbate ion, (Pb(OH)6)2−:
PbO2 + 2 NaOH + 2 H2O → Na2(Pb(OH)6)
It also responds in the melt with simple oxides, yielding M4(PbO4) orthoplumbates. Lead dioxide electrodes are used on positive plates in lead storage batteries in which lead dioxide accumulates. Lead dioxide reacts with hot acids due to the volatility of its Pb4+ cation, converting to a more stable Pb2+ state and freeing oxygen:
2 PbO2 + 2 H2SO4 → 2 PbSO4 + 2 H2O + O2
2 PbO2 + 4 HNO3 → 2 Pb(NO3)2 + 2 H2O + O2
PbO2 + 4 HCl → PbCl2 + 2 H2O + Cl2
By oxidizing an alkaline slurry of lead monoxide with chlorine, sodium hypochlorite, or bleaching powder, lead dioxide is produced. Like metals, lead dioxide has special potential for electrodes, and it can be polarized both anodically and cathodically in electrolytes. It can also be prepared by fusing a mixture of sodium nitrate and sodium chlorate with lead monoxide. There is a dual-action of lead dioxide electrodes, which is the involvement of both lead and oxygen ions in electrochemical reactions. When engaged in a fire, these compounds can intensify burning. Some may burn quickly; some may explode from heat or pollution. Therefore, using harder substrates such as titanium, niobium, tantalum, or graphite and depositing PbO2 on them from lead(II) nitrate in static or flowing nitric acid is an alternative process.
Information Sources: