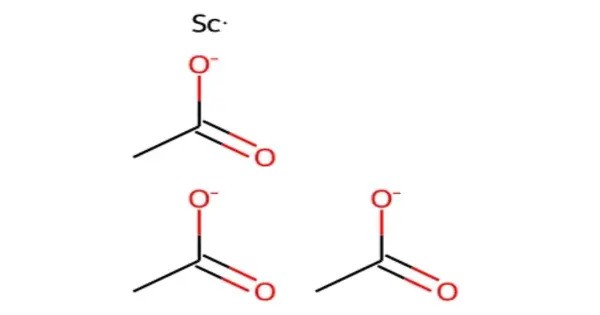
Scandium acetate is an compound, with the chemical formula of Sc(CH3COO)3. It exists in the anhydrous and the hydrate forms. It can be obtained by reacting scandium hydroxide or scandium oxide with acetic acid. It is a colorless, water-soluble solid. It decomposes into scandium oxide at high temperature. It can be used to prepare other scandium-containing materials.
The structure of the anhydrous form was determined by X-ray crystallography. It consists of a chain of octahedral Sc(III) centers linked by bridging acetate ligands.
Properties
Scandium acetate is relatively stable under normal conditions but can be reactive when exposed to certain conditions. It can undergo reactions with other compounds in solution, particularly in the presence of acids or bases, due to the acetate ions.
The melting point of scandium acetate is around 150°C. However, this may vary slightly depending on purity and exact composition. The molar mass of scandium acetate (Sc(C₂H₃O₂)₃) is approximately 234.97 g/mol. It has been explored for use as a catalyst in chemical reactions, especially in organic synthesis. It is also used in certain polymerization reactions. It is stable under normal storage conditions but should be kept away from extreme heat and reactive chemicals to prevent degradation or unwanted reactions.
Preparation
Scandium acetate is not typically found naturally in its pure form. Instead, it is synthetically produced from scandium compounds like scandium oxide (Sc₂O₃). Scandium can be extracted from ores such as thortveitite (Sc2Si2O7) or bazzite (a rare scandium-bearing mineral), and scandium salts (like scandium chloride) can then be reacted with acetic acid to form scandium acetate.
Natural Occurrence
Scandium itself is a rare earth element, and while it is found in trace amounts in the Earth’s crust, it is not typically found in high concentrations. Scandium is often extracted from minerals that contain trace amounts of this metal, rather than in large quantities.
In the Laboratory
Scandium acetate is mainly used in laboratories or industrial applications rather than occurring naturally in large quantities.
Applications
- In Chemical Synthesis: As a source of scandium ions, scandium acetate is used in various chemical reactions, particularly as a catalyst in organic reactions or in the production of certain polymers.
- In Research: Researchers use scandium acetate for studying the properties of scandium in different chemical environments. It is valuable in experiments where the scandium ion plays a role in catalysis or material science.
- Material Science: Scandium compounds, including scandium acetate, are also studied in the field of materials science for their potential applications in high-performance alloys, particularly in aluminum alloys, where scandium improves strength and corrosion resistance.